Page 353 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 353
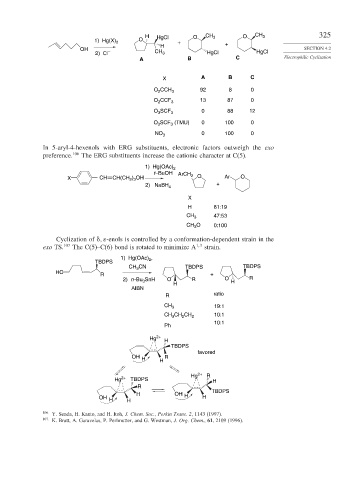
H HgCl O CH 3 O CH 3 325
1) Hg(X) 2 O +
H +
OH SECTION 4.2
2) Cl – CH 3 HgCl HgCl
A B C Electrophilic Cyclization
X A B C
O CCH 3 92 8 0
2
O 2 CCF 3 13 87 0
O SCF 3 0 88 12
3
O SCF (TMU) 0 100 0
3
3
NO 3 0 100 0
In 5-aryl-4-hexenols with ERG substituents, electronic factors outweigh the exo
preference. 106 The ERG substituents increase the cationic character at C(5).
1) Hg(OAc) 2
t–BuOH
X CH CH(CH ) OH ArCH 2 O Ar O
2 3
2) NaBH 4 +
X
H 81:19
CH 3 47:53
CH O 0:100
3
Cyclization of -enols is controlled by a conformation-dependent strain in the
exo TS. 107 The C(5)–C(6) bond is rotated to minimize A 1 3 strain.
1) Hg(OAc) ,
TBDPS 2
CH CN TBDPS TBDPS
3
HO
R +
2) n -Bu SnH 3 O R O H R
H
AIBN
R ratio
CH 3 19:1
CH CH CH 2 10:1
2
3
10:1
Ph
Hg 2+
H
TBDPS
favored
OH H H R
Hg 2+ R
Hg 2+ TBDPS H
R
TBDPS
H OH H
OH H H H
106 Y. Senda, H. Kanto, and H. Itoh, J. Chem. Soc., Perkin Trans. 2, 1143 (1997).
107
K. Bratt, A. Garavelas, P. Perlmutter, and G. Westman, J. Org. Chem., 61, 2109 (1996).