Page 350 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 350
322
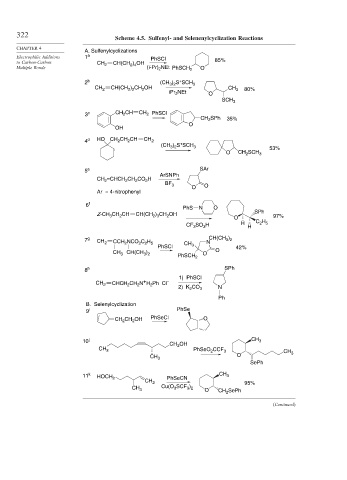
Scheme 4.5. Sulfenyl- and Selenenylcyclization Reactions
CHAPTER 4
A. Sulfenylcyclizations
Electrophilic Additions 1 a PhSCl
to Carbon-Carbon CH 2 CH(CH ) OH 85%
2 4
Multiple Bonds (i-Pr) 2 NEt PhSCH 2 O
+
2 b (CH ) S SCH 3
3 2
CH 2 CH(CH ) CH OH CH 3 80%
2
2 2
iPr NEt O
2
SCH 3
3 c CH 2 CH CH 2 PhSCl
CH 2 SPh 35%
O
OH
2
4 d HO CH 2 CH CH CH 2
+
(CH 3 ) 2 S SCH 3
53%
O CH SCH 3
2
5 e SAr
ArSNPh
CH =CHCH CH CO H
2
2
2
2
BF 3 O
O
Ar = 4-nitrophenyl
6 f
PhS N O
Z-CH CH CH CH(CH ) CH OH O SPh 97%
2 3
2
2
3
2
SO H H C H 5
CF 3 3 H
7 g CH 2 CCH NCO C H N CH(CH )
3 2
2
2 2 5
PhSCl CH 3 42%
CH 3 CH(CH ) PhSCH 2 O O
3 2
8 h SPh
1) PhSCl
+
CH 2 CHCH 2 CH 2 N H 2 Ph Cl –
2) K 2 CO 3 N
Ph
B. Selenylcyclization
9 i PhSe
CH 2 CH 2 OH PhSeCl O
j CH 3
CH OH
10
2
CH 3 PhSeO CCF 3 CH
2
CH 3 O 3
SePh
11 k HOCH 2 PhSeCN CH 3
CH 2 95%
Cu(O 3 SCF )
CH 3 3 2
O CH SePh
2
(Continued)