Page 351 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 351
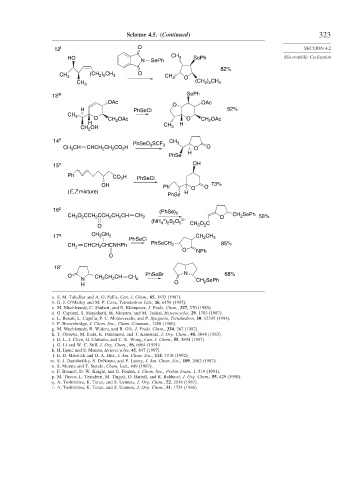
Scheme 4.5. (Continued) 323
12 l O SECTION 4.2
CH
HO 3 SePh Electrophilic Cyclization
N SePh
82%
CH 3 (CH ) CH 3 O CH 3 O
2 5
) CH
CH 3 (CH 2 5 3
13 m SePh
OAc OAc
O
H PhSeCl 52%
CH 3
O CH 2 OAc O CH OAc
H CH H 2
CH 2 OH 3
14 n PhSeO SCF CH 3
CH 3 CH CHCH CH CO H 3 3 O O
2
2
2
H
PhSe
15 o OH
Ph CO H PhSeCl
2
OH Ph O 73%
(E,Z mixture) H O
PhSe
16 p (PhSe)
CH O CCH CCH CH CH CH 2 2 O CH 2 SePh 58%
2
2
2
2
3
+
(NH ) S O 5 2–
4
2 2
2
3
O CH O C
17 q CH CH 2 CH CH 3
3
2
PhSeCl
CH 2 CHCH CHCNHPh PhSeCH 2 85%
2
O NPh
O
18 r
O CH 2 CH 2 CH PhSeBr N 68%
N CH 2 CH SePh
H O 2
a. S. M. Tuladhar and A. G. Fallis, Can. J. Chem., 65, 1833 (1987).
b. G. J. O’Malley and M. P. Cava, Tetrahedron Lett., 26, 6159 (1985).
c. M. Muehlstaedt, C. Shubert, and E. Kleinpeter, J. Prakt. Chem., 327, 270 (1985).
d. G. Capozzi, S. Menichetti, M. Nicastro, and M. Taddei, Heterocycles, 29, 1703 (1987).
e. L. Benati, L. Capella, P. C. Montevecchi, and P. Spagnolo, Tetrahedron, 50, 12395 (1994).
f. P. Brownbridge, J. Chem. Soc., Chem. Commun., 1280 (1980).
g. M. Muehlstaedt, R. Widera, and B. Olk, J. Prakt. Chem., 324, 362 (1982).
h. T. Ohsawa, M. Ihara, K. Fukumoto, and T. Kametani, J. Org. Chem., 48, 3644 (1983).
i. D. L. J. Clive, G. Chittattu, and C. K. Wong, Can. J. Chem., 55, 3894 (1987).
j. G. Li and W. C. Still, J. Org. Chem., 56, 6964 (1991).
k. H. Inoue and S. Murata, Heterocycles, 45, 847 (1997).
l. E. D. Mihelich and G. A. Hite, J. Am. Chem. Soc., 114, 7318 (1992).
m. S. J. Danishefsky, S. DeNinno, and P. Lartey, J. Am. Chem. Soc., 109, 2082 (1987).
n. S. Murata and T. Suzuki, Chem. Lett., 849 (1987).
o. F. Bennett, D. W. Knight, and G. Fenton, J. Chem. Soc., Perkin Trans. 1, 519 (1991).
p. M. Tiecco, L. Testaferri, M. Tingoli, D. Bartoli, and R. Balducci, J. Org. Chem., 55, 429 (1990).
q. A. Toshimitsu, K. Terao, and S. Uemura, J. Org. Chem., 52, 2018 (1987).
r. A. Toshimitsu, K. Terao, and S. Uemura, J. Org. Chem., 51, 1724 (1986).