Page 352 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 352
324 is mediated by dimethyl(methylthio)sulfonium tetrafluoroborate. Entries 3 and 4 are
other examples of 5-exo cyclizations. Entries 5 and 6 involve use of sulfenamides as
CHAPTER 4
the electrophiles. Entry 7 shows the cyclization of a carbamate involving the carbonyl
Electrophilic Additions oxygen. Entry 8 is an 5-endo aminocyclization.
to Carbon-Carbon
Multiple Bonds Part B of Scheme 4.5 gives some examples of cyclizations induced by selenium
electrophiles. Entries 9 to 13 are various selenyletherifications. All exhibit anti stereo-
chemistry. Entries 14 and 15 are selenyllactonizations. Entries 17 and 18 involve amido
groups as the internal nucleophile. Entry 17 is an 5-exo cyclization in which the amido
oxygen is the more reactive nucleophilic site, leading to an iminolactone. Geometric
factors favor N-cyclization in the latter case.
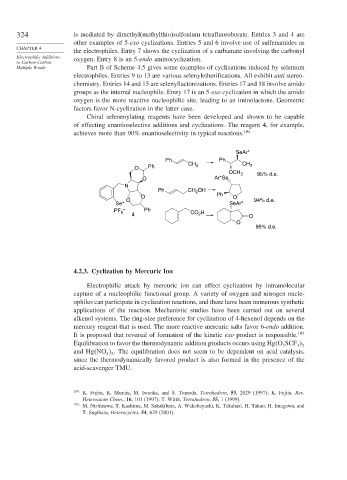
Chiral selenenylating reagents have been developed and shown to be capable
of effecting enantioselective additions and cyclizations. The reagent 4, for example,
achieves more than 90% enantioselectivity in typical reactions. 104
SeAr*
Ph Ph
CH 3 CH 3
O Ph
OCH 3 95% d.e.
O Ar*Se
N
Ph CH OH
2
O Ph O
+ O 94% d.e.
Se SeAr*
PF 6 – Ph H
4 CO 2 O
O
95% d.e.
4.2.3. Cyclization by Mercuric Ion
Electrophilic attack by mercuric ion can effect cyclization by intramolecular
capture of a nucleophilic functional group. A variety of oxygen and nitrogen nucle-
ophiles can participate in cyclization reactions, and there have been numerous synthetic
applications of the reaction. Mechanistic studies have been carried out on several
alkenol systems. The ring-size preference for cyclization of 4-hexenol depends on the
mercury reagent that is used. The more reactive mercuric salts favor 6-endo addition.
It is proposed that reversal of formation of the kinetic exo product is responsible. 105
Equilibration to favor the thermodynamic addition products occurs using Hg O SCF
3 2
3
and Hg NO . The equilibration does not seem to be dependent on acid catalysis,
3 2
since the thermodynamically favored product is also formed in the presence of the
acid-scavenger TMU.
104 K. Fujita, K. Murata, M. Iwaoka, and S. Tomoda, Tetrahedron, 53, 2029 (1997); K. Fujita, Rev.
Heteroatom Chem., 16, 101 (1997); T. Wirth, Tetrahedron, 55, 1 (1999).
105
M. Nishizawa, T. Kashima, M. Sakakibara, A. Wakabayashi, K. Takahasi, H. Takao, H. Imagawa, and
T. Sugihara, Heterocycles, 54, 629 (2001).