Page 355 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 355
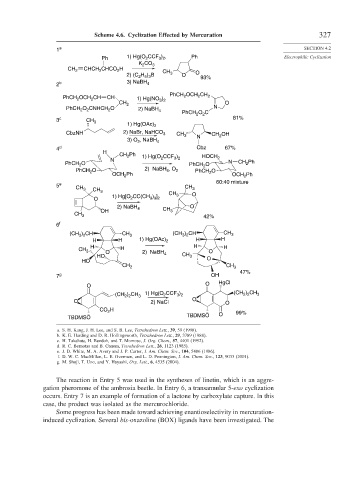
Scheme 4.6. Cyclization Effected by Mercuration 327
1 a SECTION 4.2
Ph 1) Hg(O 2 CCF ) , Ph Electrophilic Cyclization
3 2
K CO 3
2
CH 2 CHCH CHCO H CH
2
2
2) (C H ) B 3 O O 93%
2 5 3
2 b 3) NaBH 4
PhCH OCH CH
PhCH 2 OCH 2 CH CH 1) Hg(NO ) 2 2 2
3 2
CH 2 O
PhCH O CNHCH O 2) NaBH 4 PhCH 2 O 2 C N
2
2
2
3 c CH 3 81%
1) Hg(OAc) 2
CbzNH 2) NaBr, NaHCO 3 CH 3 N CH OH
2
3) O 2 , NaBH 4
4 d Cbz 67%
H
CH Ph CCF ) HOCH
2
N 1) Hg(O 2 3 2 2 N CH Ph
PhCH O PhCH O 2
2
2
O 2) NaBH , O
PhCH 2 4 2 PhCH 2 O
OCH Ph OCH Ph
2
2
60:40 mixture
5 e
CH 3 CH 3 CH 3
CC(CH ) ] CH 3 O
O 1) Hg[O 2 3 3 2
2) NaBH O
OH 4 CH 3
CH 3 42%
6 f
(CH ) CH CH 3 (CH ) CH CH 3
3 2
3 2
H H 1) Hg(OAc) 2 H H
H H H H
CH 3 O 2) NaBH 4 O
HO CH 3 O
HO
CH 2 CH 3
47%
7 g OH
O HgCl
O
(CH ) CH 3 1) Hg(O CCF ) (CH ) CH 3
2 2
2
3 2
2 2
O 2) NaCl O O
H
CO 2 99%
TBDMSO TBDMSO O
a. S. H. Kang, J. H. Lee, and S. B. Lee, Tetrahedron Lett., 39, 59 (1998).
b. K. E. Harding and D. R. Hollingsworth, Tetrahedron Lett., 29, 3789 (1988).
c. H. Takahata, H. Bandoh, and T. Momose, J. Org. Chem., 57, 4401 (1992).
d. R. C. Bernotas and B. Ganem, Tetrahedron Lett., 26, 1123 (1985).
e. J. D. White, M. A. Avery and J. P. Carter, J. Am. Chem. Soc., 104, 5486 (1986).
f. D. W. C. MacMillan, L. E. Overman, and L. D. Pennington, J. Am. Chem. Soc., 123, 9033 (2001).
g. M. Shoji, T. Uno, and Y. Hayashi, Org. Lett., 6, 4535 (2004).
The reaction in Entry 5 was used in the syntheses of linetin, which is an aggre-
gation pheromone of the ambrosia beetle. In Entry 6, a transannular 5-exo cyclization
occurs. Entry 7 is an example of formation of a lactone by carboxylate capture. In this
case, the product was isolated as the mercurochloride.
Some progress has been made toward achieving enantioselectivity in mercuration-
induced cyclization. Several bis-oxazoline (BOX) ligands have been investigated. The