Page 370 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 370
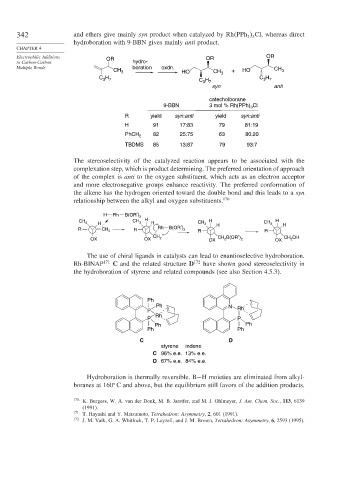
342 and ethers give mainly syn product when catalyzed by Rh PPh Cl, whereas direct
3 3
hydroboration with 9-BBN gives mainly anti product.
CHAPTER 4
Electrophilic Additions OR OR OR
to Carbon-Carbon hydro-
Multiple Bonds boration oxdn. CH
CH 3 HO CH 3 + HO 3
C H C H C H
3 7
3 7
3 7
syn anti
catecholborane
9-BBN 3 mol % Rh(PPh) 3 Cl
R yield syn:anti yield syn:anti
H 91 17:83 79 81:19
82 25:75 63 80:20
PhCH 2
TBDMS 85 13:87 79 93:7
The stereoselectivity of the catalyzed reaction appears to be associated with the
complexation step, which is product determining. The preferred orientation of approach
of the complex is anti to the oxygen substituent, which acts as an electron acceptor
and more electronegative groups enhance reactivity. The preferred conformation of
the alkene has the hydrogen oriented toward the double bond and this leads to a syn
relationship between the alkyl and oxygen substituents. 170
H Rh B(OR′) 2
CH 3 H CH 3 H H CH 3 H CH 3 H
R CH 2 R Rh B(OR′) 2 R H R H
CH CH B(OR′) CH OH
OX OX 2 OX 2 2 OX 2
The use of chiral ligands in catalysts can lead to enantioselective hydroboration.
Rh-BINAP 171 C and the related structure D 172 have shown good stereoselectivity in
the hydroboration of styrene and related compounds (see also Section 4.5.3).
Ph
Ph N
P Rh
Rh
P P
Ph Ph
Ph Ph
C D
styrene indene
C 96% e.e. 13% e.e.
D 67% e.e. 84% e.e.
Hydroboration is thermally reversible. B−H moieties are eliminated from alkyl-
boranes at 160 C and above, but the equilibrium still favors of the addition products.
170
K. Burgess, W. A. van der Donk, M. B. Jarstfer, and M. J. Ohlmeyer, J. Am. Chem. Soc., 113, 6139
(1991).
171 T. Hayashi and Y. Matsumoto, Tetrahedron: Asymmetry, 2, 601 (1991).
172
J. M. Valk, G. A. Whitlock, T. P. Layzell, and J. M. Brown, Tetrahedron: Asymmetry, 6, 2593 (1995).