Page 428 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 428
In synthesis, the principal factors that affect the choice of a reducing agent are 401
selectivity among functional groups (chemoselectivity) and stereoselectivity. Chemo-
selectivity can involve two issues. One may wish to effect a partial reduction of a SECTION 5.3
particular functional group or it may be necessary to reduce one group in preference Group III
Hydride-Donor Reagents
78
to another. In the sections that follow, we consider some synthetically useful partial
and selective reductions.
5.3.1.1. Partial Reduction of Carboxylic Acid Derivatives. One of the more difficult
partial reductions is the conversion of a carboxylic acid derivative to an aldehyde
without overreduction to the alcohol. Aldehydes are inherently more reactive than
acids or esters, so the challenge is to stop the reduction at the aldehyde stage.
Several approaches have been used to achieve this objective. One is to replace
some of the hydrogens in the hydride with more bulky groups, thus modifying
reactivity by steric factors. Lithium tri-t-butoxyaluminum hydride is an example of
this approach. 79 Sodium tri-t-butoxyaluminum hydride can be used to reduce acid
80
chlorides to aldehydes without overreduction to the alcohol. The excellent solubility
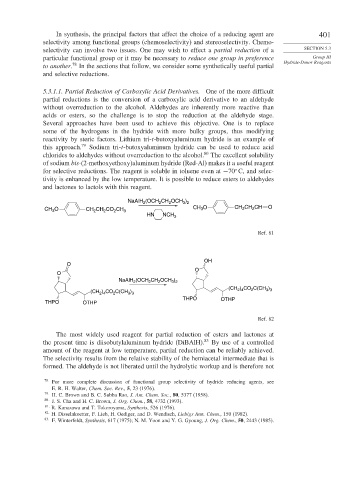
of sodium bis-(2-methoxyethoxy)aluminum hydride (Red-Al) makes it a useful reagent
for selective reductions. The reagent is soluble in toluene even at −70 C, and selec-
tivity is enhanced by the low temperature. It is possible to reduce esters to aldehydes
and lactones to lactols with this reagent.
(OCH CH OCH )
NaAlH 2 2 2 3 2
CH O CH 2 CH CO CH 3 CH 3 O CH CH CH O
2
2
2
2
3
HN NCH 3
Ref. 81
OH
O
O
O
NaAlH (OCH CH OCH )
2
2
3 2
2
(CH ) CO C(CH )
(CH ) CO C(CH ) 2 4 2 3 3
3 3
2 4
2
THPO OTHP
THPO OTHP
Ref. 82
The most widely used reagent for partial reduction of esters and lactones at
the present time is diisobutylaluminum hydride (DiBAlH). 83 By use of a controlled
amount of the reagent at low temperature, partial reduction can be reliably achieved.
The selectivity results from the relative stability of the hemiacetal intermediate that is
formed. The aldehyde is not liberated until the hydrolytic workup and is therefore not
78
For more complete discussion of functional group selectivity of hydride reducing agents, see
E. R. H. Walter, Chem. Soc. Rev., 5, 23 (1976).
79 H. C. Brown and B. C. Subba Rao, J. Am. Chem. Soc., 80, 5377 (1958).
80
J. S. Cha and H. C. Brown, J. Org. Chem., 58, 4732 (1993).
81
R. Kanazawa and T. Tokoroyama, Synthesis, 526 (1976).
82 H. Disselnkoetter, F. Lieb, H. Oediger, and D. Wendisch, Liebigs Ann. Chem., 150 (1982).
83
F. Winterfeldt, Synthesis, 617 (1975); N. M. Yoon and Y. G. Gyoung, J. Org. Chem., 50, 2443 (1985).