Page 429 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 429
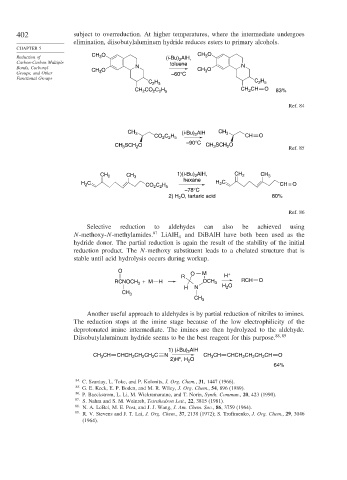
402 subject to overreduction. At higher temperatures, where the intermediate undergoes
elimination, diisobutylaluminum hydride reduces esters to primary alcohols.
CHAPTER 5
CH O CH 3 O
Reduction of 3 (i-Bu) AlH,
2
Carbon-Carbon Multiple toluene
Bonds, Carbonyl CH O N CH O N
Groups, and Other 3 –60°C 3
Functional Groups
C H C H
2 5
2 5
CO C H CH O
CH 2 2 2 5 CH 2 83%
Ref. 84
CH 3 (i-Bu) AlH CH 3
CO C H 2 CH O
2 2 5
–90°C
CH SCH O CH SCH O Ref. 85
3
2
3
2
CH 2 CH 3 1)(i-Bu) 2 AlH, CH 2 CH 3
hexane
H C CO C H H C CH = O
2
2
2 2 5
–78°C
2) H O, tartaric acid 80%
2
Ref. 86
Selective reduction to aldehydes can also be achieved using
N-methoxy-N-methylamides. 87 LiAlH and DiBAlH have both been used as the
4
hydride donor. The partial reduction is again the result of the stability of the initial
reduction product. The N-methoxy substituent leads to a chelated structure that is
stable until acid hydrolysis occurs during workup.
O O M
R H +
RCNOCH + M H OCH 3 RCH O
3
H N H O
2
CH 3
CH 3
Another useful approach to aldehydes is by partial reduction of nitriles to imines.
The reduction stops at the imine stage because of the low electrophilicity of the
deprotonated imine intermediate. The imines are then hydrolyzed to the aldehyde.
Diisobutylaluminum hydride seems to be the best reagent for this purpose. 88 89
AlH
1) (i-Bu) 2
CH CH CHCH 2 CH CH C N CH CH CHCH CH CH CH O
2
3
2
2
2
2
3
+
2)H , H O
2
64%
84
C. Szantay, L. Toke, and P. Kolonits, J. Org. Chem., 31, 1447 (1966).
85 G. E. Keck, E. P. Boden, and M. R. Wiley, J. Org. Chem., 54, 896 (1989).
86 P. Baeckstrom, L. Li, M. Wickramaratne, and T. Norin, Synth. Commun., 20, 423 (1990).
87
S. Nahm and S. M. Weinreb, Tetrahedron Lett., 22, 3815 (1981).
88 N. A. LeBel, M. E. Post, and J. J. Wang, J. Am. Chem. Soc., 86, 3759 (1964).
89
R. V. Stevens and J. T. Lai, J. Org. Chem., 37, 2138 (1972); S. Trofimenko, J. Org. Chem., 29, 3046
(1964).