Page 434 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 434
O H R O O 407
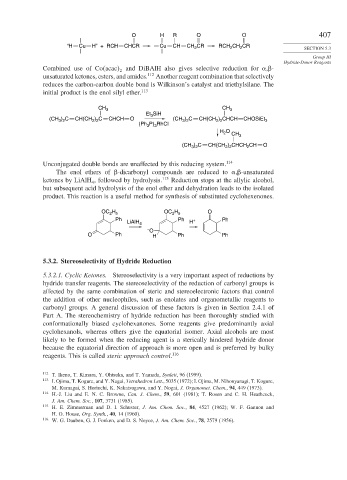
“H Cu H” + RCH CHCR Cu CH CH CR RCH CH CR SECTION 5.3
2
2
2
Group III
Hydride-Donor Reagents
Combined use of Co acac and DiBAlH also gives selective reduction for , -
2
unsaturated ketones, esters, and amides. 112 Another reagent combination that selectively
reduces the carbon-carbon double bond is Wilkinson’s catalyst and triethylsilane. The
initial product is the enol silyl ether. 113
CH 3 CH 3
Et SiH
3
(CH ) C CH(CH ) C CHCH O (CH ) C CH(CH ) CHCH CHOSiEt 3
3 2
2 2
3 2
2 2
(Ph P) RhCl
3
3
H O CH 3
2
(CH ) C CH(CH ) CHCH CH O
2
2 2
3 2
Unconjugated double bonds are unaffected by this reducing system. 114
The enol ethers of -dicarbonyl compounds are reduced to , -unsaturated
ketones by LiAlH , followed by hydrolysis. 115 Reduction stops at the allylic alcohol,
4
but subsequent acid hydrolysis of the enol ether and dehydration leads to the isolated
product. This reaction is a useful method for synthesis of substituted cyclohexenones.
OC H OC H O
2 5
2 5
Ph Ph + Ph
LiAlH 4 H
– O
O Ph H Ph Ph
5.3.2. Stereoselectivity of Hydride Reduction
5.3.2.1. Cyclic Ketones. Stereoselectivity is a very important aspect of reductions by
hydride transfer reagents. The stereoselectivity of the reduction of carbonyl groups is
affected by the same combination of steric and stereoelectronic factors that control
the addition of other nucleophiles, such as enolates and organometallic reagents to
carbonyl groups. A general discussion of these factors is given in Section 2.4.1 of
Part A. The stereochemistry of hydride reduction has been thoroughly studied with
conformationally biased cyclohexanones. Some reagents give predominantly axial
cyclohexanols, whereas others give the equatorial isomer. Axial alcohols are most
likely to be formed when the reducing agent is a sterically hindered hydride donor
because the equatorial direction of approach is more open and is preferred by bulky
reagents. This is called steric approach control. 116
112 T. Ikeno, T. Kimura, Y. Ohtsuka, and T. Yamada, Synlett, 96 (1999).
113
I. Ojima, T. Kogure, and Y. Nagai, Tetrahedron Lett., 5035 (1972); I. Ojima, M. Nihonyanagi, T. Kogure,
M. Kumagai, S. Horiuchi, K. Nakatsugawa, and Y. Nogai, J. Organomet. Chem., 94, 449 (1973).
114 H.-J. Liu and E. N. C. Browne, Can. J. Chem., 59, 601 (1981); T. Rosen and C. H. Heathcock,
J. Am. Chem. Soc., 107, 3731 (1985).
115 H. E. Zimmerman and D. I. Schuster, J. Am. Chem. Soc., 84, 4527 (1962); W. F. Gannon and
H. O. House, Org. Synth., 40, 14 (1960).
116
W. G. Dauben, G. J. Fonken, and D. S. Noyce, J. Am. Chem. Soc., 78, 2579 (1956).