Page 439 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 439
2
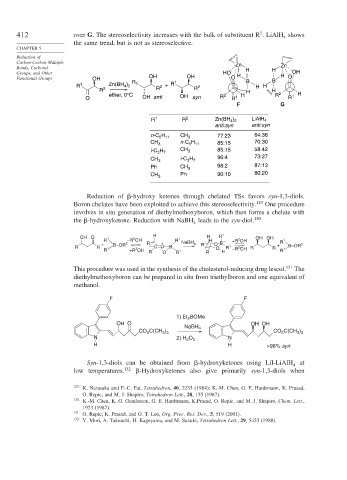
412 over G. The stereoselectivity increases with the bulk of substituent R . LiAlH shows
4
the same trend, but is not as stereoselective.
CHAPTER 5
Reduction of
Carbon-Carbon Multiple Zn
Bonds, Carbonyl H H Zn
Groups, and Other HO H H OH
Functional Groups OH OH 1 OH O B B O
R 1 2 Zn(BH ) R 1 R 2 + R R 2 H H
4 2
R H H
ether, 0°C 2 H R 2 1 H
O OH anti OH syn R R 1 R
F G
R 1 R 2 Zn(BH ) LiAlH 4
4 2
anti:syn anti:syn
H
n-C 5 11 CH 3 77:23 64:36
CH 3 n-C H 85:15 70:30
5 11
i-C H CH 3 85:15 58:42
3 7
CH 3 i-C H 96:4 73:27
3 7
Ph CH 3 98:2 87:13
CH 3 Ph 90:10 80:20
Reduction of -hydroxy ketones through chelated TSs favors syn-1,3-diols.
Boron chelates have been exploited to achieve this stereoselectivity. 129 One procedure
involves in situ generation of diethylmethoxyboron, which then forms a chelate with
the -hydroxyketone. Reduction with NaBH leads to the syn-diol. 130
4
H 1
OH O H R OH OH
2
2
R 1 –R OH R 1 H – +R OH R 1
+ B–OR 2 R + NaBH 4 R O B 2
R R C O B O 1 2 R R + B–OR
2
R 1 +R OH R O R 1 R H R –R OH R 1
This procedure was used in the synthesis of the cholesterol-reducing drug lescol. 131 The
diethylmethoxyboron can be prepared in situ from triethylboron and one equivalent of
methanol.
F F
1) Et BOMe
2
OH O OH OH
NaBH 4
C(CH ) CO C(CH )
CO 2 3 3 2 3 3
N 2) H 2 O 2 N
H H >98% syn
Syn-1,3-diols can be obtained from -hydroxyketones using LiI-LiAlH 4 at
low temperatures. 132 -Hydroxyketones also give primarily syn-1,3-diols when
129 K. Narasaka and F.-C. Pai, Tetrahedron, 40, 2233 (1984); K.-M. Chen, G. E. Hardtmann, K. Prasad,
O. Repic, and M. J. Shapiro, Tetrahedron Lett., 28, 155 (1987).
130
K.-M. Chen, K. G. Gunderson, G. E. Hardtmann, K.Prasad, O. Repic, and M. J. Shapiro, Chem. Lett.,
1923 (1987).
131 O. Repic, K. Prasad, and G. T. Lee, Org. Proc. Res. Dev., 5, 519 (2001).
132
Y. Mori, A. Takeuchi, H. Kageyama, and M. Suzuki, Tetrahedron Lett., 29, 5423 (1988).