Page 435 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 435
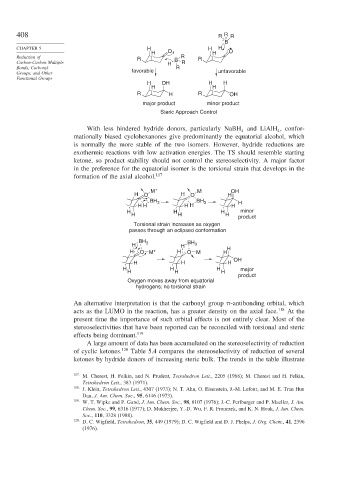
408 R R R
– B
CHAPTER 5 H H H
H O H O
Reduction of R – R R
Carbon-Carbon Multiple H B R
Bonds, Carbonyl R
Groups, and Other favorable unfavorable
Functional Groups
H OH H H
H H
R H R OH
major product minor product
Steric Approach Control
With less hindered hydride donors, particularly NaBH and LiAlH , confor-
4
4
mationally biased cyclohexanones give predominantly the equatorial alcohol, which
is normally the more stable of the two isomers. However, hydride reductions are
exothermic reactions with low activation energies. The TS should resemble starting
ketone, so product stability should not control the stereoselectivity. A major factor
in the preference for the equatorial isomer is the torsional strain that develops in the
formation of the axial alcohol. 117
M + M OH
H O H O H
BH 3 BH 3 H
H H H H H
H H H minor
H H H
product
Torsional strain increases as oxygen
passes through an eclipsed conformation
BH 3 BH
H H 3
H O M + H O M H H
OH
H H H
H H H major
H H H
product
Oxygen moves away from equatorial
hydrogens; no torsional strain
An alternative interpretation is that the carbonyl group -antibonding orbital, which
acts as the LUMO in the reaction, has a greater density on the axial face. 118 At the
present time the importance of such orbital effects is not entirely clear. Most of the
stereoselectivities that have been reported can be reconciled with torsional and steric
effects being dominant. 119
A large amount of data has been accumulated on the stereoselectivity of reduction
of cyclic ketones. 120 Table 5.4 compares the stereoselectivity of reduction of several
ketones by hydride donors of increasing steric bulk. The trends in the table illustrate
117 M. Cherest, H. Felkin, and N. Prudent, Tetrahedron Lett., 2205 (1968); M. Cherest and H. Felkin,
Tetrahedron Lett., 383 (1971).
118
J. Klein, Tetrahedron Lett., 4307 (1973); N. T. Ahn, O. Eisenstein, J.-M. Lefour, and M. E. Tran Huu
Dau, J. Am. Chem. Soc., 95, 6146 (1973).
119 W. T. Wipke and P. Gund, J. Am. Chem. Soc., 98, 8107 (1976); J.-C. Perlburger and P. Mueller, J. Am.
Chem. Soc., 99, 6316 (1977); D. Mukherjee, Y.-D. Wu, F. R. Fronczek, and K. N. Houk, J. Am. Chem.
Soc., 110, 3328 (1988).
120
D. C. Wigfield, Tetrahedron, 35, 449 (1979); D. C. Wigfield and D. J. Phelps, J. Org. Chem., 41, 2396
(1976).