Page 440 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 440
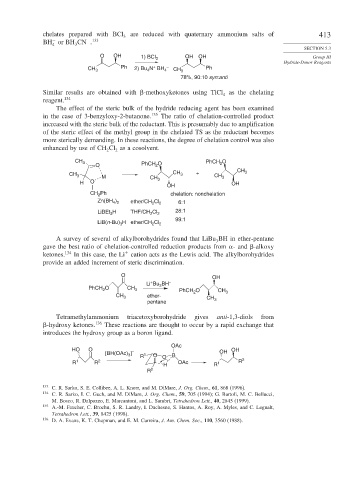
chelates prepared with BCl are reduced with quaternary ammonium salts of 413
3
− 133
BH or BH CN .
−
4 3
SECTION 5.3
O OH OH OH Group III
1) BCl 3
Hydride-Donor Reagents
+
CH 3 Ph 2) Bu N BH 4 – CH 3 Ph
4
78%, 90:10 syn:anti
Similar results are obtained with -methoxyketones using TiCl as the chelating
4
reagent. 134
The effect of the steric bulk of the hydride reducing agent has been examined
in the case of 3-benzyloxy-2-butanone. 135 The ratio of chelation-controlled product
increased with the steric bulk of the reductant. This is presumably due to amplification
of the steric effect of the methyl group in the chelated TS as the reductant becomes
more sterically demanding. In these reactions, the degree of chelation control was also
enhanced by use of CH Cl as a cosolvent.
2
2
CH 3 PhCH O PhCH O
2
O 2
CH
CH 3 M CH 3 + CH 3 3
H O CH 3 OH
OH
CH Ph chelation: nonchelation
2
Zn(BH ) ether/CH 2 Cl 2 6:1
4 2
H THF/CH Cl 28:1
LiBEt 3 2 2
99:1
LiB(n-Bu) 3 H ether/CH 2 Cl 2
A survey of several of alkylborohydrides found that LiBu BH in ether-pentane
3
gave the best ratio of chelation-controlled reduction products from - and -alkoxy
+
ketones. 134 In this case, the Li cation acts as the Lewis acid. The alkylborohydrides
provide an added increment of steric discrimination.
O
OH
+
Li Bu BH –
3
PhCH O CH 3 PhCH O CH
2
CH 3 ether- 2 CH 3
pentane 3
Tetramethylammonium triacetoxyborohydride gives anti-1,3-diols from
-hydroxy ketones. 136 These reactions are thought to occur by a rapid exchange that
introduces the hydroxy group as a boron ligand.
OAc
HO O OH
[BH(OAc) ] – R 1 O O B OH
3
R 1 R 2 OAc 1 R 2
H R
R 2
133
C. R. Sarko, S. E. Collibee, A. L. Knorr, and M. DiMare, J. Org. Chem., 61, 868 (1996).
134 C. R. Sarko, I. C. Guch, and M. DiMare, J. Org. Chem., 59, 705 (1994); G. Bartoli, M. C. Bellucci,
M. Bosco, R. Dalpozzo, E. Marcantoni, and L. Sambri, Tetrahedron Lett., 40, 2845 (1999).
135 A.-M. Faucher, C. Brochu, S. R. Landry, I. Duchesne, S. Hantos, A. Roy, A. Myles, and C. Legualt,
Tetrahedron Lett., 39, 8425 (1998).
136
D. A. Evans, K. T. Chapman, and E. M. Carreira, J. Am. Chem. Soc., 110, 3560 (1988).