Page 444 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 444
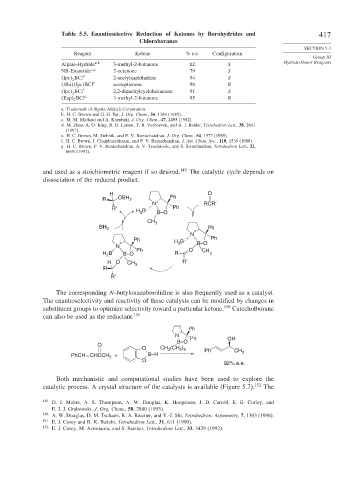
Table 5.5. Enantioselective Reduction of Ketones by Borohydrides and 417
Chloroboranes
SECTION 5.3
Reagent Ketone % e.e. Configuration
Group III
Alpine-Hydride a b 3-methyl-2-butanone 62 S Hydride-Donor Reagents
NB-Enantride a c 2-octanone 79 S
Ipc 2 BCl d 2-acetylnaphthalene 94 S
tBu Ipc BCl e acetophenone 96 R
Ipc 2 BCl f 2,2-dimethylcyclohexanone 91 S
Eap 2 BCl g 3-methyl-2-butanone 95 R
a. Trademark of Sigma-Aldrich Corporation.
b. H. C. Brown and G. G. Pai, J. Org. Chem., 50, 1384 (1985).
c. M. M. Midland and A. Kozubski, J. Org. Chem., 47, 2495 (1982).
d. M. Zhao, A. O. King, R. D. Larsen, T. R. Verhoeven, and A. J. Reider, Tetrahedron Lett., 38, 2641
(1997).
e. H. C. Brown, M. Srebnik, and P. V. Ramachandran, J. Org. Chem., 54, 1577 (1989).
f. H. C. Brown, J. Chandrasekharan, and P. V. Ramachandran, J. Am. Chem. Soc., 110, 1539 (1988).
g. H. C. Brown, P. V. Ramachandran, A. V. Teodorovic, and S. Swaminathan, Tetrahedron Lett., 32,
6691 (1991).
and used as a stoichiometric reagent if so desired. 149 The catalytic cycle depends on
dissociation of the reduced product.
H O
R OBH 2 Ph
N + RCR′
R′ – Ph
H B B–O
3
CH 3
BH 3 Ph
+
N
Ph – Ph
+ H B B–O
3
N
Ph O CH
H 2 B B–O R 3
H O CH 3 R′
R
R′
The corresponding N-butyloxazaborolidine is also frequently used as a catalyst.
The enantioselectivity and reactivity of these catalysts can be modified by changes in
substituent groups to optimize selectivity toward a particular ketone. 150 Catecholborane
can also be used as the reductant. 151
Ph
N Ph
B–O OH
O
O CH (CH ) Ph CH
2 3
3
PhCH = CHCCH + B–H 3
3
O
92% e.e.
Both mechanistic and computational studies have been used to explore the
catalytic process. A crystal structure of the catalysts is available (Figure 5.7). 152 The
149
D. J. Mahre, A. S. Thompson, A. W. Douglas, K. Hoogsteen, J. D. Carroll, E. G. Corley, and
E. J. J. Grabowski, J. Org. Chem., 58, 2880 (1993).
150 A. W. Douglas, D. M. Tschaen, R. A. Reamer, and Y.-J. Shi, Tetrahedron: Asymmetry, 7, 1303 (1996).
151 E. J. Corey and R. K. Bakshi, Tetrahedron Lett., 31, 611 (1990).
152
E. J. Corey, M. Azimiaora, and S. Sarshar, Tetrahedron Lett., 33, 3429 (1992).