Page 447 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 447
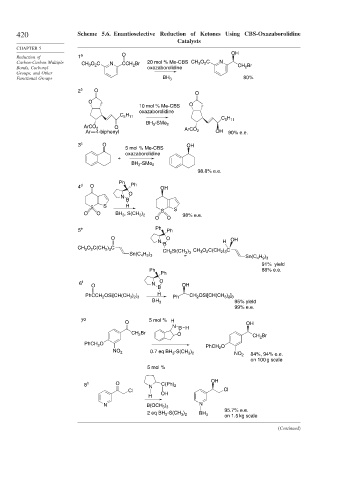
420 Scheme 5.6. Enantioselective Reduction of Ketones Using CBS-Oxazaborolidine
Catalysts
CHAPTER 5
Reduction of 1 a O OH
Carbon-Carbon Multiple CH 3 O 2 C N CCH 2 Br 20 mol % Me-CBS CH 3 O 2 C N
Bonds, Carbonyl oxazaborolidine CH 2 Br
Groups, and Other
Functional Groups BH 3 80%
2 b O
O
O
10 mol % Me-CBS O
oxazaborolidine
C 5 H 11
C 5 H 11
BH 3 -SMe 2
O
ArCO 2
Ar 4-biphenyl ArCO 2 OH 90% e.e.
3 c O OH
5 mol % Me-CBS
oxazaborolidine
+
BH 3 -SMe 2
98.8% e.e.
Ph
4 d O Ph OH
O
N
B
S S H S
O O BH 3 , S(CH 3 ) 2 S 98% e.e.
O O
5 e Ph Ph
O O OH
N H
B
CH 3 O 2 C(CH 2 ) 3 C
CH 2 Si(CH 3 ) 3 CH 3 O 2 C(CH 2 ) 3 C
Sn(C 4 H 9 ) 3
Sn(C 4 H 9 ) 3
91% yield
Ph 88% e.e.
Ph
6 f N O
O B OH
H
PhCCH 2 OSi[CH(CH 3 ) 2 ] 3 Ph CH 2 OSi[CH(CH 3 ) 2 ] 3
BH 3 95% yield
99% e.e.
7 g O 5 mol % H OH
N B H
CH 2 Br O CH 2 Br
PhCH 2 O
PhCH 2 O
NO 2 0.7 eq BH 3 -S(CH 3 ) 2
NO 2 84%, 94% e.e.
on 100 g scale
5 mol %
OH
8 h O N C(Ph) 2
Cl Cl
H OH
N B(OCH 3 ) 3 N
95.7% e.e.
2 eq BH 3 -S(CH 3 ) 2 BH 3
on 1.5 kg scale
(Continued)