Page 442 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 442
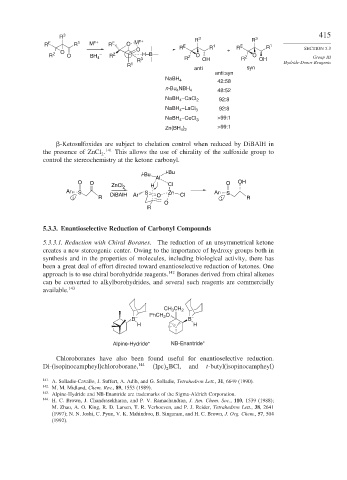
R 3 R 3 R 3 415
R E R 1 M n+ R E O M n+ E 1 E 1
O R R + R R SECTION 5.3
O
R Z O BH 4 – R Z R 3 H–B R Z O OH R Z O OH Group III
R 1 Hydride-Donor Reagents
anti syn
anti:syn
NaBH 4
42:58
n-Bu NBH 4 48:52
4
NaBH –CaCl 2 92:8
4
NaBH –LaCl 3 92:8
4
NaBH –CeCl 3 >99:1
4
Zn(BH ) >99:1
4 2
-Ketosulfoxides are subject to chelation control when reduced by DiBAlH in
the presence of ZnCl . 141 This allows the use of chirality of the sulfoxide group to
2
control the stereochemistry at the ketone carbonyl.
i-Bu
i-Bu
Al
O O O OH
ZnCl 2 H Cl
Ar S S Zn Ar S
: R DiBAlH Ar O Cl : R
O
R
5.3.3. Enantioselective Reduction of Carbonyl Compounds
5.3.3.1. Reduction with Chiral Boranes. The reduction of an unsymmetrical ketone
creates a new stereogenic center. Owing to the importance of hydroxy groups both in
synthesis and in the properties of molecules, including biological activity, there has
been a great deal of effort directed toward enantioselective reduction of ketones. One
approach is to use chiral borohydride reagents. 142 Boranes derived from chiral alkenes
can be converted to alkylborohydrides, and several such reagents are commercially
available. 143
CH CH 2
3
PhCH O
B – 2 B –
H H
Alpine-Hydride* NB-Enantride*
Chloroboranes have also been found useful for enantioselective reduction.
Di-(isopinocampheyl)chloroborane, 144 Ipc BCl, and t-butyl(isopinocampheyl)
2
141 A. Solladie-Cavallo, J. Suffert, A. Adib, and G. Solladie, Tetrahedron Lett., 31, 6649 (1990).
142
M. M. Midland, Chem. Rev., 89, 1553 (1989).
143 Alpine-Hydride and NB-Enantride are trademarks of the Sigma-Aldrich Corporation.
144
H. C. Brown, J. Chandrasekharan, and P. V. Ramachandran, J. Am. Chem. Soc., 110, 1539 (1988);
M. Zhao, A. O. King, R. D. Larsen, T. R. Verhoeven, and P. J. Reider, Tetrahedron Lett., 38, 2641
(1997); N. N. Joshi, C. Pyun, V. K. Mahindroo, B. Singaram, and H. C. Brown, J. Org. Chem., 57, 504
(1992).