Page 441 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 441
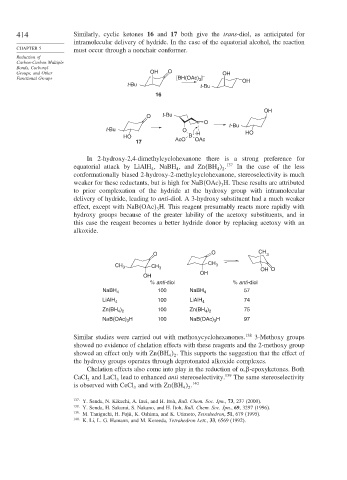
414 Similarly, cyclic ketones 16 and 17 both give the trans-diol, as anticipated for
intramolecular delivery of hydride. In the case of the equatorial alcohol, the reaction
CHAPTER 5 must occur through a nonchair conformer.
Reduction of
Carbon-Carbon Multiple
Bonds, Carbonyl
Groups, and Other OH O OH
Functional Groups [BH(OAc) 3 ] – OH
t-Bu t- Bu
16
OH
O t- Bu
O
t- Bu
t-Bu O HO
HO B H
17 AcO OAc
In 2-hydroxy-2,4-dimethylcyclohexanone there is a strong preference for
equatorial attack by LiAlH , NaBH , and Zn BH . 137 In the case of the less
4
4 2
4
conformationally biased 2-hydroxy-2-methylcyclohexanone, stereoselectivity is much
weaker for these reductants, but is high for NaB OAc H. These results are attributed
3
to prior complexation of the hydride at the hydroxy group with intramolecular
delivery of hydride, leading to anti-diol. A 3-hydroxy substituent had a much weaker
effect, except with NaB OAc H. This reagent presumably reacts more rapidly with
3
hydroxy groups because of the greater lability of the acetoxy substituents, and in
this case the reagent becomes a better hydride donor by replacing acetoxy with an
alkoxide.
O O CH 3
CH
CH 3 CH 3 3 OH O
OH
OH
% anti-diol % anti-diol
NaBH 4 100 NaBH 4 57
LiAlH 4 100 LiAlH 4 74
Zn(BH ) 100 Zn(BH ) 75
4 2
4 2
NaB(OAc) H 100 NaB(OAc) H 97
3
3
Similar studies were carried out with methoxycyclohexanones. 138 3-Methoxy groups
showed no evidence of chelation effects with these reagents and the 2-methoxy group
showed an effect only with Zn BH . This supports the suggestion that the effect of
4 2
the hydroxy groups operates through deprotonated alkoxide complexes.
Chelation effects also come into play in the reduction of , -epoxyketones. Both
CaCl and LaCl lead to enhanced anti stereoselectivity. 139 The same stereoselectivity
2
3
is observed with CeCl and with Zn BH . 140
3
4 2
137
Y. Senda, N. Kikuchi, A. Inui, and H. Itoh, Bull. Chem. Soc. Jpn., 73, 237 (2000).
138
Y. Senda, H. Sakurai, S. Nakano, and H. Itoh, Bull. Chem. Soc. Jpn., 69, 3297 (1996).
139 M. Taniguchi, H. Fujii, K. Oshima, and K. Utimoto, Tetrahedron, 51, 679 (1995).
140
K. Li, L. G. Hamann, and M. Koreeda, Tetrahedron Lett., 33, 6569 (1992).