Page 436 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 436
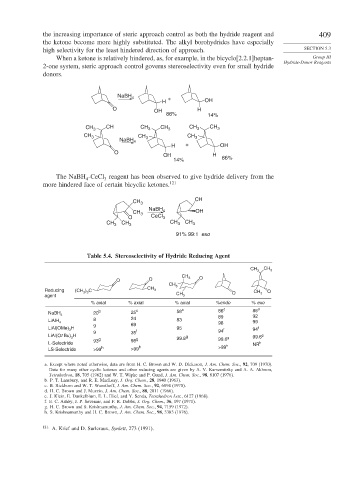
the increasing importance of steric approach control as both the hydride reagent and 409
the ketone become more highly substituted. The alkyl borohydrides have especially
high selectivity for the least hindered direction of approach. SECTION 5.3
When a ketone is relatively hindered, as, for example, in the bicyclo[2.2.1]heptan- Group III
Hydride-Donor Reagents
2-one system, steric approach control governs stereoselectivity even for small hydride
donors.
NaBH 4
H + OH
O OH H
86% 14%
CH CH CH
CH 3 CH 3 3 CH 3 3
CH 3 CH 3 CH 3
NaBH 4
H + OH
O
OH H
14% 86%
The NaBH -CeCl reagent has been observed to give hydride delivery from the
3
4
more hindered face of certain bicyclic ketones. 121
CH 3 CH
NaBH
CH 3 4 OH
O CeCl 3
CH 3 CH 3 CH 3 CH 3
91% 99:1 exo
Table 5.4. Stereoselectivity of Hydride Reducing Agent
CH CH
3 3
CH O
O O 3
CH
CH 3
Reducing (CH ) C 3 O CH O
3 3
agent CH 3 3
% axial % axial % axial %endo % exo
b c 58 c 86 d 86 d
20 25
NaBH 4 89 92
LiAlH 4 8 24 83 98 99
9 69
LiAl(OMe) H 95 94 f
3 f 94 f
9 35
H g
LiAl(Ot Bu) 3 g g 99.8 g 99.6 g 99.6
93 98
L-Selectride NR h
LS-Selectride >99 h >99 h >99 h
a. Except where noted otherwise, data are from H. C. Brown and W. D. Dickason, J. Am. Chem. Soc., 92, 709 (1970).
Data for many other cyclic ketones and other reducing agents are given by A. V. Kamernitzky and A. A. Akhrem,
Tetrahedron, 18, 705 (1962) and W. T. Wipke and P. Gund, J. Am. Chem. Soc., 98, 8107 (1976).
b. P. T. Lansbury, and R. E. MacLeay, J. Org. Chem., 28, 1940 (1963).
c. B. Rickborn and W. T. Wuesthoff, J. Am. Chem. Soc., 92, 6894 (1970).
d. H. C. Brown and J. Muzzio, J. Am. Chem. Soc., 88, 2811 (1966).
e. J. Klein, E. Dunkelblum, E. L. Eliel, and Y. Senda, Tetrahedron Lett., 6127 (1968).
f. E. C. Ashby, J. P. Sevenair, and F. R. Dobbs, J. Org. Chem., 36, 197 (1971).
g. H. C. Brown and S. Krishnamurthy, J. Am. Chem. Soc., 94, 7159 (1972).
h. S. Krishnamurthy and H. C. Brown, J. Am. Chem. Soc., 98, 3383 (1976).
121
A. Krief and D. Surleraux, Synlett, 273 (1991).