Page 432 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 432
+ – 405
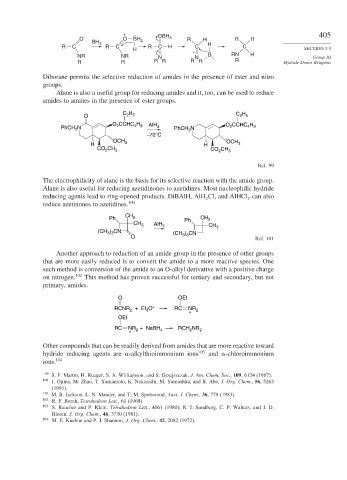
O O BH OBH 2 R H R H
BH 3 2
R C R C R C H C H C SECTION 5.3
H
+
NR NR N N B RN H Group III
R R R R R R R Hydride-Donor Reagents
Diborane permits the selective reduction of amides in the presence of ester and nitro
groups.
Alane is also a useful group for reducing amides and it, too, can be used to reduce
amides to amines in the presence of ester groups.
C H C H
O 2 5 2 5
O CCHC H AlH O CCHC H
4 9
2
PhCH N 3 PhCH N 2 4 9
2
2
–70°C
OCH OCH
H 3 H 3
CO CH 3 CO CH 3
2
2
Ref. 99
The electrophilicity of alane is the basis for its selective reaction with the amide group.
Alane is also useful for reducing azetidinones to azetidines. Most nucleophilic hydride
reducing agents lead to ring-opened products. DiBAlH, AlH Cl, and AlHCl can also
2
2
reduce azetinones to azetidines. 100
Ph CH 3 Ph CH 3
CH 3 AlH 3 CH 3
(CH ) CN (CH ) CN
3 3
O 3 3 Ref. 101
Another approach to reduction of an amide group in the presence of other groups
that are more easily reduced is to convert the amide to a more reactive species. One
such method is conversion of the amide to an O-alkyl derivative with a positive charge
on nitrogen. 102 This method has proven successful for tertiary and secondary, but not
primary, amides.
O OEt
RCNR + Et O + RC NR 2
2
3
+
OEt
RC NR 2 + NaBH 4 RCH NR 2
2
+
Other compounds that can be readily derived from amides that are more reactive toward
hydride reducing agents are -alkylthioimmonium ions 103 and -chloroimmonium
ions. 104
99
S. F. Martin, H. Rueger, S. A. Williamson, and S. Grzejszczak, J. Am. Chem. Soc., 109, 6124 (1987).
100 I. Ojima, M. Zhao, T. Yamamoto, K. Nakanishi, M. Yamashita, and R. Abe, J. Org. Chem., 56, 5263
(1991).
101 M. B. Jackson, L. N. Mander, and T. M. Spotswood, Aust. J. Chem., 36, 779 (1983).
102
R. F. Borch, Tetrahedron Lett., 61 (1968).
103 S. Raucher and P. Klein, Tetrahedron Lett., 4061 (1980); R. J. Sundberg, C. P. Walters, and J. D.
Bloom, J. Org. Chem., 46, 3730 (1981).
104
M. E. Kuehne and P. J. Shannon, J. Org. Chem., 42, 2082 (1972).