Page 657 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 657
632 7.1.2.3. Preparation by Halogen-Metal Exchange. Halogen-metal exchange is
another important method for preparation of organolithium reagents. The reaction
CHAPTER 7 proceeds in the direction of forming the more stable organolithium reagent, that is, the
Organometallic one derived from the more acidic organic compound. Thus, by use of the very basic
Compounds of Group I
and II Metals organolithium compounds n-butyl- or t-butyllithium, halogen substituents at more
2
acidic sp carbons are readily exchanged to give the corresponding lithium compound.
Halogen-metal exchange is particularly useful for converting aryl and alkenyl halides
to the corresponding lithium compounds.
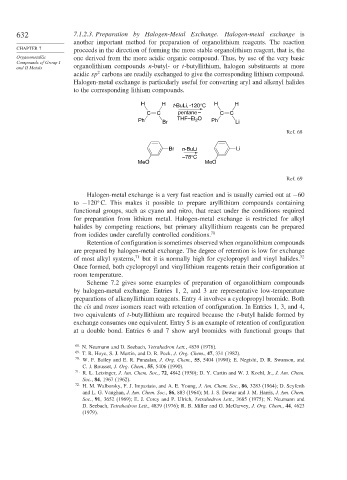
H H t-BuLi, -120°C H H
C C pentane – C C
Ph Br THF–Et O Ph Li
2
Ref. 68
Br n -BuLi Li
–78°C
MeO MeO
Ref. 69
Halogen-metal exchange is a very fast reaction and is usually carried out at −60
to −120 C. This makes it possible to prepare aryllithium compounds containing
functional groups, such as cyano and nitro, that react under the conditions required
for preparation from lithium metal. Halogen-metal exchange is restricted for alkyl
halides by competing reactions, but primary alkyllithium reagents can be prepared
from iodides under carefully controlled conditions. 70
Retention of configuration is sometimes observed when organolithium compounds
are prepared by halogen-metal exchange. The degree of retention is low for exchange
of most alkyl systems, 71 but it is normally high for cyclopropyl and vinyl halides. 72
Once formed, both cyclopropyl and vinyllithium reagents retain their configuration at
room temperature.
Scheme 7.2 gives some examples of preparation of organolithium compounds
by halogen-metal exchange. Entries 1, 2, and 3 are representative low-temperature
preparations of alkenyllithium reagents. Entry 4 involves a cyclopropyl bromide. Both
the cis and trans isomers react with retention of configuration. In Entries 1, 3, and 4,
two equivalents of t-butyllithium are required because the t-butyl halide formed by
exchange consumes one equivalent. Entry 5 is an example of retention of configuration
at a double bond. Entries 6 and 7 show aryl bromides with functional groups that
68
N. Neumann and D. Seebach, Tetrahedron Lett., 4839 (1976).
69 T. R. Hoye, S. J. Martin, and D. R. Peck, J. Org. Chem., 47, 331 (1982).
70
W. F. Bailey and E. R. Punzalan, J. Org. Chem., 55, 5404 (1990); E. Negishi, D. R. Swanson, and
C. J. Rousset, J. Org. Chem., 55, 5406 (1990).
71 R. L. Letsinger, J. Am. Chem. Soc., 72, 4842 (1950); D. Y. Curtin and W. J. Koehl, Jr., J. Am. Chem.
Soc., 84, 1967 (1962).
72
H. M. Walborsky, F. J. Impastato, and A. E. Young, J. Am. Chem. Soc., 86, 3283 (1964); D. Seyferth
and L. G. Vaughan, J. Am. Chem. Soc., 86, 883 (1964); M. J. S. Dewar and J. M. Harris, J. Am. Chem.
Soc., 91, 3652 (1969); E. J. Corey and P. Ulrich, Tetrahedron Lett., 3685 (1975); N. Neumann and
D. Seebach, Tetrahedron Lett., 4839 (1976); R. B. Miller and G. McGarvey, J. Org. Chem., 44, 4623
(1979).