Page 655 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 655
630
c
CHAPTER 7
Organometallic c c
Compounds of Group I F c o
c 1.466Å 1.427Å
and II Metals c c
1.877Å 1.920Å
c
c Li Li
2.270Å c 2.220Å
c
c 1.403Å 1.671Å c 1.411Å 1.640Å
2.088Å 2.090Å
1.502Å
1.512Å
c
c
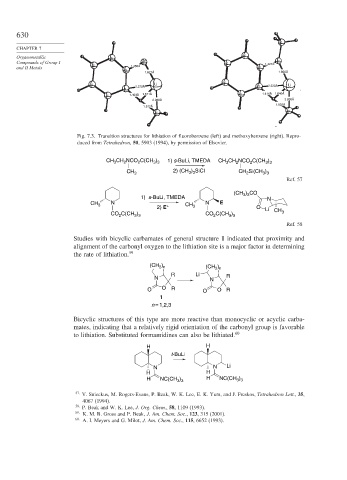
Fig. 7.3. Transition structures for lithiation of fluorobenzene (left) and methoxybenzene (right). Repro-
duced from Tetrahedron, 50, 5903 (1994), by permission of Elsevier.
CH NCO C(CH )
CH 3 2 2 3 3 1) s-BuLi, TMEDA CH CH NCO C(CH )
3 3
2
2
3
) SiCl
CH 3 2) (CH 3 3 CH Si(CH )
3 3
2
Ref. 57
(CH ) CO
3 3
1) s-BuLi, TMEDA N
CH 3 N + CH N E
2) E 3 O Li CH
C(CH ) CO C(CH ) 3
CO 2 3 3 2 3 3
Ref. 58
Studies with bicyclic carbamates of general structure 1 indicated that proximity and
alignment of the carbonyl oxygen to the lithiation site is a major factor in determining
the rate of lithiation. 59
)
(CH 2 n (CH )
2 n
R Li
N N R
O O R O O R
1
n = 1,2,3
Bicyclic structures of this type are more reactive than monocyclic or acyclic carba-
mates, indicating that a relatively rigid orientation of the carbonyl group is favorable
to lithiation. Substituted formamidines can also be lithiated. 60
H H
t-BuLi
N N Li
H H
H NC(CH ) H NC(CH )
3 3
3 3
57
V. Snieckus, M. Rogers-Evans, P. Beak, W. K. Lee, E. K. Yum, and J. Freskos, Tetrahedron Lett., 35,
4067 (1994).
58 P. Beak and W. K. Lee, J. Org. Chem., 58, 1109 (1993).
59 K. M. B. Gross and P. Beak, J. Am. Chem. Soc., 123, 315 (2001).
60
A. I. Meyers and G. Milot, J. Am. Chem. Soc., 115, 6652 (1993).