Page 651 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 651
626 in turn, are solvated with ether molecules. 39 Phenyllithium is tetrameric in cyclo-
hexane and a mixture of monomer and dimer in THF. 40 Chelating ligands
CHAPTER 7 41
such as tetramethylethylenediamine (TMEDA) reduce the degree of aggregation.
Organometallic Strong donor molecules such as hexamethylphosphorotriamide (HMPA) and N,N-
Compounds of Group I
and II Metals dimethylpropyleneurea (DMPU) also lead to more dissociated and more reactive
organolithium reagents. 42 NMR studies on phenyllithium show that TMEDA, other
polyamine ligands, HMPA, and DMPU favor monomeric solvated species. 43
CH 3
N
) ]
O P[N(CH 3 2 3 O
N
HMPA CH 3 DMPU
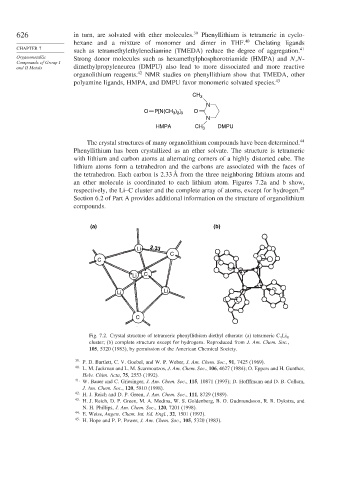
The crystal structures of many organolithium compounds have been determined. 44
Phenyllithium has been crystallized as an ether solvate. The structure is tetrameric
with lithium and carbon atoms at alternating corners of a highly distorted cube. The
lithium atoms form a tetrahedron and the carbons are associated with the faces of
the tetrahedron. Each carbon is 2.33 Å from the three neighboring lithium atoms and
an ether molecule is coordinated to each lithium atom. Figures 7.2a and b show,
respectively, the Li–C cluster and the complete array of atoms, except for hydrogen. 45
Section 6.2 of Part A provides additional information on the structure of organolithium
compounds.
(a) (b)
Li 2.33
C
C
Li C
Li Li
C
Fig. 7.2. Crystal structure of tetrameric phenyllithium diethyl etherate: (a) tetrameric C 4 Li 4
cluster; (b) complete structure except for hydrogens. Reproduced from J. Am. Chem. Soc.,
105, 5320 (1983), by permission of the American Chemical Society.
39 P. D. Bartlett, C. V. Goebel, and W. P. Weber, J. Am. Chem. Soc., 91, 7425 (1969).
40
L. M. Jackman and L. M. Scarmoutzos, J. Am. Chem. Soc., 106, 4627 (1984); O. Eppers and H. Gunther,
Helv. Chim. Acta, 75, 2553 (1992).
41 W. Bauer and C. Griesinger, J. Am. Chem. Soc., 115, 10871 (1993); D. Hofffmann and D. B. Collum,
J. Am. Chem. Soc., 120, 5810 (1998).
42
H. J. Reich and D. P. Green, J. Am. Chem. Soc., 111, 8729 (1989).
43 H. J. Reich, D. P. Green, M. A. Medina, W. S. Goldenberg, B. O. Gudmundsson, R. R. Dykstra, and
N. H. Phillips, J. Am. Chem. Soc., 120, 7201 (1998).
44 E. Weiss, Angew. Chem. Int. Ed. Engl., 32, 1501 (1993).
45
H. Hope and P. P. Power, J. Am. Chem. Soc., 105, 5320 (1983).