Page 654 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 654
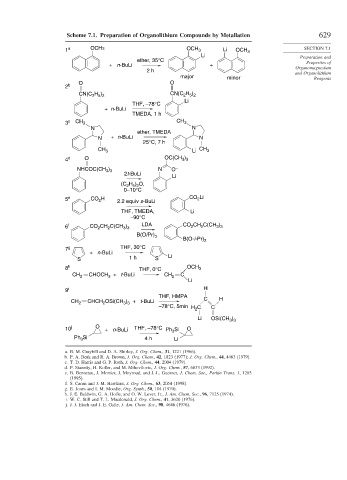
Scheme 7.1. Preparation of Organolithium Compounds by Metallation 629
1 a OCH3 OCH 3 Li OCH 3 SECTION 7.1
Li Preparation and
ether, 35°C Properties of
+ n-BuLi + Organomagnesium
2 h and Organolithium
major minor Reagents
O O
2 b
CN(C H ) CN(C H )
2 5 2
2 5 2
Li
THF, –78°C
+ n-BuLi
TMEDA, 1 h
3 c CH 3 CH 3
N N
ether, TMEDA
N + n-BuLi N
25°C, 7 h
CH 3 Li CH 3
)
4 d O OC(CH 3 3
NHCOC(CH ) N O –
3 3
2t-BuLi
Li
H ) O,
(C 2 5 2
0–10°C
5 e CO 2 H CO Li
2
2.2 equiv s-BuLi
THF, TMEDA, Li
–90°C
2
6 f CO 2 CH 2 C(CH 3 ) 3 LDA CO CH C(CH )
2
3 3
B(Oi Pr) 3
B(O-i-Pr) 2
7 g THF, 30°C
+ n-BuLi
S 1 h S Li
8 h THF, 0°C OCH 3
CH 2 CHOCH 3 + t-BuLi CH 2 C
Li
9 i H
THF, HMPA
CH 2 CHCH 2 OSi(CH ) + t-BuLi C H
3 3
–78°C, 5min H C C
2
Li OSi(CH 3 3
)
10 j O + n-BuLi THF, –78°C Ph Si O
3
Ph Si 4 h Li
3
a. B. M. Graybill and D. A. Shirley, J. Org. Chem., 31, 1221 (1966).
b. P. A. Beak and R. A. Brown, J. Org. Chem., 42, 1823 (1977); J. Org. Chem., 44, 4463 (1979).
c. T. D. Harris and G. P. Roth, J. Org. Chem., 44, 2004 (1979).
d. P. Stanetty, H. Koller, and M. Mihovilovic, J. Org. Chem., 57, 6833 (1992).
e. B. Bennetau, J. Mortier, J. Moyroud, and J.-L. Guesnet, J. Chem. Soc., Perkin Trans. 1, 1265
(1995).
f. S. Caron and J. M. Hawkins, J. Org. Chem., 63, 2054 (1998).
g. E. Jones and I. M. Moodie, Org. Synth., 50, 104 (1970).
h. J. E. Baldwin, G. A. Hofle, and O. W. Lever, Jr., J. Am. Chem. Soc., 96, 7125 (1974).
i. W. C. Still and T. L. Macdonald, J. Org. Chem., 41, 3620 (1976).
j. J. J. Eisch and J. E. Galle, J. Am. Chem. Soc., 98, 4646 (1976).