Page 649 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 649
624
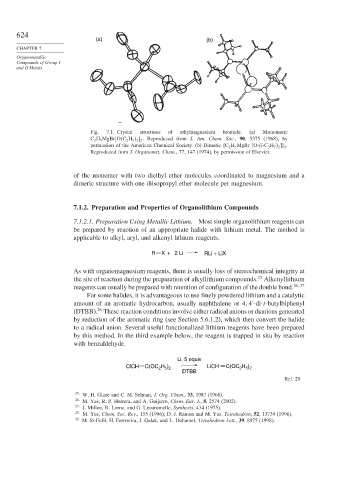
(a) (b)
CHAPTER 7
Organometallic
Compounds of Group I
and II Metals
Fig. 7.1. Crystal structures of ethylmagnesium bromide: (a) Monomeric
C 2 H 5 MgBr O C 2 H 5 2 2 . Reproduced from J. Am. Chem. Soc., 90, 5375 (1968), by
permission of the American Chemical Society. (b) Dimeric C 2 H 5 MgBr [O-(i-C 3 H 7 2 .
2
Reproduced from J. Organomet. Chem., 77, 147 (1974), by permission of Elsevier.
of the monomer with two diethyl ether molecules coordinated to magnesium and a
dimeric structure with one diisopropyl ether molecule per magnesium.
7.1.2. Preparation and Properties of Organolithium Compounds
7.1.2.1. Preparation Using Metallic Lithium. Most simple organolithium reagents can
be prepared by reaction of an appropriate halide with lithium metal. The method is
applicable to alkyl, aryl, and alkenyl lithium reagents.
R X + 2 Li RLi + LiX
As with organomagnesium reagents, there is usually loss of stereochemical integrity at
25
the site of reaction during the preparation of alkyllithium compounds. Alkenyllithium
reagents can usually be prepared with retention of configuration of the double bond. 26 27
For some halides, it is advantageous to use finely powdered lithium and a catalytic
amount of an aromatic hydrocarbon, usually naphthalene or 4 4 -di-t-butylbiphenyl
28
(DTBB). These reaction conditions involve either radical anions or dianions generated
by reduction of the aromatic ring (see Section 5.6.1.2), which then convert the halide
to a radical anion. Several useful functionalized lithium reagents have been prepared
by this method. In the third example below, the reagent is trapped in situ by reaction
with benzaldehyde.
Li, 5 equiv
H )
H )
ClCH C(OC 2 5 2 LiCH C(OC 2 5 2
DTBB
Ref. 29
25 W. H. Glaze and C. M. Selman, J. Org. Chem., 33, 1987 (1968).
26
M. Yus, R. P. Herrera, and A. Guijarro, Chem. Eur. J., 8, 2574 (2002).
27
J. Millon, R. Lorne, and G. Linstrumelle, Synthesis, 434 (1975).
28 M. Yus, Chem. Soc. Rev., 155 (1996); D. J. Ramon and M. Yus, Tetrahedron, 52, 13739 (1996).
29
M. Si-Fofil, H. Ferrrerira, J. Galak, and L. Duhamel, Tetrahedron Lett., 39, 8975 (1998).