Page 645 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 645
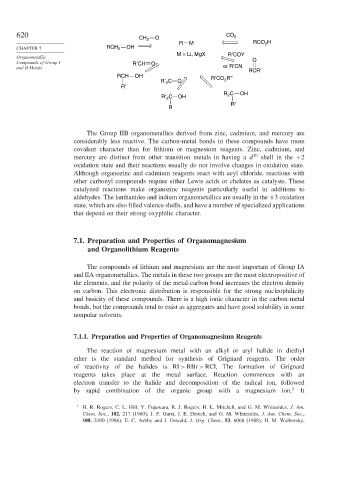
620 CO 2
CH 2 O
R M RCO H
2
CHAPTER 7 RCH 2 OH
M = Li, MgX R′COY
Organometallic
Compounds of Group I R′CH O O
and II Metals or R′CN RCR′
RCH OH R′CO R″
R′ C O 2
2
R′
R 2 C OH
R′ 2 C OH
R′
R
The Group IIB organometallics derived from zinc, cadmium, and mercury are
considerably less reactive. The carbon-metal bonds in these compounds have more
covalent character than for lithium or magnesium reagents. Zinc, cadmium, and
mercury are distinct from other transition metals in having a d 10 shell in the +2
oxidation state and their reactions usually do not involve changes in oxidation state.
Although organozinc and cadmium reagents react with acyl chloride, reactions with
other carbonyl compounds require either Lewis acids or chelates as catalysts. These
catalyzed reactions make organozinc reagents particularly useful in additions to
aldehydes. The lanthanides and indium organometallics are usually in the +3 oxidation
state, which are also filled valence shells, and have a number of specialized applications
that depend on their strong oxyphilic character.
7.1. Preparation and Properties of Organomagnesium
and Organolithium Reagents
The compounds of lithium and magnesium are the most important of Group IA
and IIA organometallics. The metals in these two groups are the most electropositive of
the elements, and the polarity of the metal-carbon bond increases the electron density
on carbon. This electronic distribution is responsible for the strong nucleophilicity
and basicity of these compounds. There is a high ionic character in the carbon-metal
bonds, but the compounds tend to exist as aggregates and have good solubility in some
nonpolar solvents.
7.1.1. Preparation and Properties of Organomagnesium Reagents
The reaction of magnesium metal with an alkyl or aryl halide in diethyl
ether is the standard method for synthesis of Grignard reagents. The order
of reactivity of the halides is RI > RBr > RCl. The formation of Grignard
reagents takes place at the metal surface. Reaction commences with an
electron transfer to the halide and decomposition of the radical ion, followed
by rapid combination of the organic group with a magnesium ion. 1 It
1
H. R. Rogers, C. L. Hill, Y. Fujuwara, R. J. Rogers, H. L. Mitchell, and G. M. Whitesides, J. Am.
Chem. Soc., 102, 217 (1980); J. F. Garst, J. E. Deutch, and G. M. Whitesides, J. Am. Chem. Soc.,
108, 2490 (1986); E. C. Ashby and J. Oswald, J. Org. Chem., 53, 6068 (1988); H. M. Walborsky,