Page 642 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 642
616
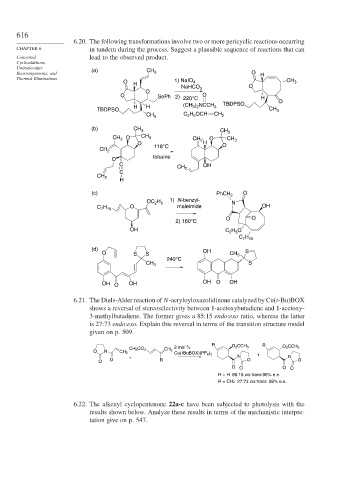
6.20. The following transformations involve two or more pericyclic reactions occurring
CHAPTER 6 in tandem during the process. Suggest a plausible sequence of reactions that can
Concerted lead to the observed product.
Cycloadditions,
Unimolecular (a) CH
Rearrangements, and 3 O H
Thermal Eliminations
O 1) NaIO 4 CH 3
H
NaHCO 3 O
O
O SePh 2) 220°C O H
H H (CH ) NCCH 3 TBDPSO O
3 2
TBDPSO CH 3
C H OCH
CH 3 2 5 CH 2
(b) CH 3 CH 3
CH 3 O CH 3 CH 2 O CH 3
O H O
CH 2 118°C
toluene
O
C OH
CH 2
C
CH 2
H
(c) PhCH 2 O
OC H 1) N-benzyl- N
2 5
C H O maleimide OH
7 15
O O
2) 160°C
OH C H O
2 5
C H
7 15
(d) OH
O S S CH 3 S
240°C
CH 3 S
OH O OH OH O OH
6.21. The Diels-Alder reaction of N-acryloyloxazolidinone catalyzed by Cu(t-Bu)BOX
shows a reversal of stereoselectivity between 1-acetoxybutadiene and 1-acetoxy-
3-methylbutadiene. The former gives a 85:15 endo:exo ratio, whereas the latter
is 27:73 endo:exo. Explain this reversal in terms of the transition structure model
given on p. 509.
R R
2 mol % O 2 CCH 3 O 2 CCH 3
O N CH 3 CO 2 CH 2
CH 2
Cu(t BuBOX)(PF 6 ) 2 +
+ N N
O R O O
O
O O O O
R = H 85:15 cis:trans 96% e.e.
R = CH3 27:73 cis:trans 98% e.e.
6.22. The alkenyl cyclopentenone 22a-c have been subjected to photolysis with the
results shown below. Analyze these results in terms of the mechanistic interpre-
tation give on p. 547.