Page 641 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 641
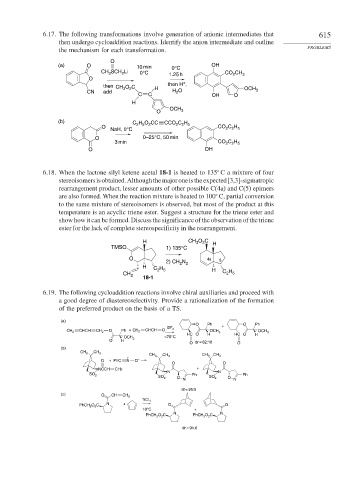
6.17. The following transformations involve generation of anionic intermediates that 615
then undergo cycloaddition reactions. Identify the anion intermediate and outline
the mechanism for each transformation. PROBLEMS
O
(a) O 10 min 0°C OH
CH 3 SCH 2 Li 0°C 1.25 h CO 2 CH 3
O
+
then CH 3 O 2 C H then H ,
CN add H 2 O OCH 3
C C OH O
H
O OCH 3
(b) C 2 H 5 O 2 CC CCO 2 C 2 H 5
O CO 2 C 2 H 5
NaH, 0°C
O 0–25°C, 50 min
3 min CO 2 C 2 H 5
O OH
6.18. When the lactone silyl ketene acetal 18-1 is heated to 135 C a mixture of four
stereoisomersisobtained.Althoughthemajoroneistheexpected[3,3]-sigmatropic
rearrangement product, lesser amounts of other possible C(4a) and C(5) epimers
are also formed. When the reaction mixture is heated to 100 C, partial conversion
to the same mixture of stereoisomers is observed, but most of the product at this
temperature is an acyclic triene ester. Suggest a structure for the triene ester and
show how it can be formed. Discuss the significance of the observation of the triene
ester for the lack of complete stereospecificity in the rearrangement.
H CH O C H
2
3
TMSO 1) 135°C
O 4a 5
2) CH N
2 2
H H
C 2 5 H
H
CH 2 C 2 5
18-1
6.19. The following cycloaddition reactions involve chiral auxiliaries and proceed with
a good degree of diastereoselectivity. Provide a rationalization of the formation
of the preferred product on the basis of a TS.
(a)
O Ph O Ph
+
BF 3
CHCH O CHCH O
CH 2 CH 2 Ph + CH 2 OCH 3 OCH 3
HC O H HC O H
–78°C
OCH 3
O H
O dr = 82:18 O
(b)
CH 3 CH 3
CH 3 CH 3 CH 3 CH 3
+
O + PhC N O –
O O
NCCH CH2 +
N N
SO 2 Ph Ph
SO 2 O SO 2 O
N N
dr = 95:5
(c) O CH CH 2
TiCl 4
PhCH 2 O 2 C N + O O
10°C +
PhCH 2 O 2 C N PhCH 2 O 2 C N
dr = 94.6