Page 640 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 640
614 CH CH CH 3
(a) 3 3 (b)
H CH 3
CHAPTER 6 H
CH 3
Concerted H
Cycloadditions, H CH O C
Unimolecular O 3 2
Rearrangements, and HO C O
Thermal Eliminations OCH 2 Ph 2
CH 2
CH O
3
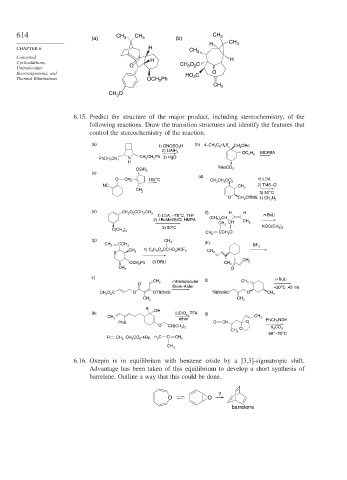
6.15. Predict the structure of the major product, including stereochemistry, of the
following reactions. Draw the transition structures and identify the features that
control the stereochemistry of the reaction.
(a) (b) 4–CH 3 C 6 H 4 S
1) ONOSO 3 H CH 2 OAc
2) LiAlH 4 MCPBA
OC 2 H 5
N CH 2 CH 2 Ph 3) HgO
PhCH 2 CH 2
H
MesCO 2
OSiR 3
(c)
(d)
O CH 2 165°C CH 3 CH 2 CO 2 1) LDA
NC CH 3 2) TMS–Cl
CH
3 3) 50°C
O CH 2 OTMS 4) CH 2 N 2
(e) CH 2 O 2 CCH 2 CH 3 (f) H H
1) LDA, –78°C, THF n-BuLi
2) t-BuMe2SiCl, HMPA (CH 3 ) 2 CH CH 3
CH 3 CH
3) 50°C KOC(CH 3 ) 3
C(CH 3 ) 3
CH 2 CCH 2 O
(g) CH 3
(h)
CH 2 CCH 3
BF 3
1) C 2 H 5 O 2 CCHO 3 SCF 3
CH 3 CH 2
S
OCH 2 Ph 2) DBU CH 2 CH 2
CH 3 O
(i) (j) n-BuLi
CH 2 intramolecular CH 2
O
Diels–Alder –20°C, 45 min
CH 3 O 2 C O OTBDMS TBDMSO O CH 3
CH 3 CH 2
R
(k) OH LiClO 4 , TFA (l)
CH 3
CH 2
ether PhCH 2 NOH
PhS O CH O
O CH(CH 3 ) 2
O K 2 CO 3
CH 3
60°–70°C
R CH 3 , CH 2 CO 2 -t-Bu, H 2 C C CH 2
CH 3
6.16. Oxepin is in equilibrium with benzene oxide by a [3,3]-sigmatropic shift.
Advantage has been taken of this equilibrium to develop a short synthesis of
barrelene. Outline a way that this could be done.
?
O O
barrelene