Page 635 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 635
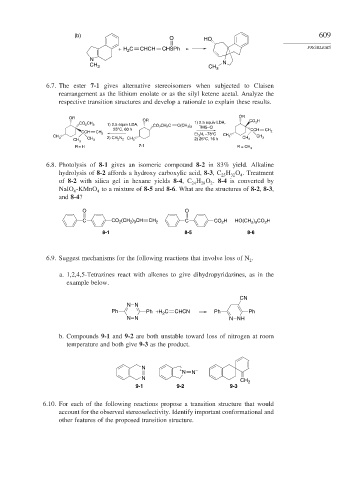
(b) 609
O HO
+ H C CHCH CHSPh PROBLEMS
2
N
CH 3 CH 3 N
6.7. The ester 7-1 gives alternative stereoisomers when subjected to Claisen
rearrangement as the lithium enolate or as the silyl ketene acetal. Analyze the
respective transition structures and develop a rationale to explain these results.
OR
OR
OR CO 2 H
1) 2.5 equiv LDA,
CO 2 CH 3 1) 2.5 equiv LDA, CO 2 CH 2 C C(CH 3 ) 2 TMS–Cl
25°C, 60 h CCH
CCH CH 2 CH 2
Et 3 N, –78°C
CH 3
CH 3 CH 3 CH 3
CH 3 2) CH 2 N 2 CH 3 2) 25°C, 16 h
CH 3
R = H 7-1 R = CH 3
6.8. Photolysis of 8-1 gives an isomeric compound 8-2 in 83% yield. Alkaline
hydrolysis of 8-2 affords a hydroxy carboxylic acid, 8-3,C H O . Treatment
25 32 4
of 8-2 with silica gel in hexane yields 8-4,C H O . 8-4 is converted by
24 28 2
NaIO -KMnO to a mixture of 8-5 and 8-6. What are the structures of 8-2, 8-3,
4
4
and 8-4?
O O
C CO 2 (CH 2 ) 9 CH CH 2 C CO 2 H HO(CH 2 ) 9 CO 2 H
8-1 8-5 8-6
6.9. Suggest mechanisms for the following reactions that involve loss of N .
2
a. 1,2,4,5-Tetrazines react with alkenes to give dihydropyridazines, as in the
example below.
CN
N N
Ph Ph +H C CHCN Ph Ph
2
NN NNH
b. Compounds 9-1 and 9-2 are both unstable toward loss of nitrogen at room
temperature and both give 9-3 as the product.
N
+ –
N N
N CH
9-1 9-2 9-3 2
6.10. For each of the following reactions propose a transition structure that would
account for the observed stereoselectivity. Identify important conformational and
other features of the proposed transition structure.