Page 632 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 632
606 (c) (d)
O
Ph O
CHAPTER 6 H Δ
CH 2 NC 90°C CH 2 CH(CH 3 ) 2
Concerted Ph Ph
Cycloadditions, H CH 3 H
Unimolecular
Rearrangements, and
Thermal Eliminations
(e) CN
CH 3 O
(CH 2 ) 4 CH CH 2 Δ
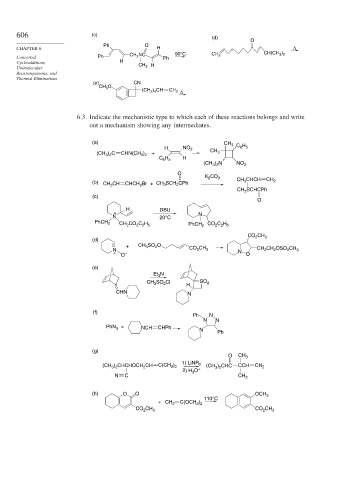
6.3. Indicate the mechanistic type to which each of these reactions belongs and write
out a mechanism showing any intermediates.
(a)
H NO 2 CH 3 C 6 H 5
(CH 3 ) 2 C CHN(CH 3 ) 2 + CH 3
H
C 6 H 5
(CH 3 ) 2 N NO 2
O
K 2 CO 3 CH 3 CHCH
(b) CH 3 CH CHCH 2 Br + CH 3 SCH 2 CPh CH 2
CH 3 SCHCPh
(c)
O
H DBU
+ N
N 20°C
PhCH 2
CH 2 CO 2 C 2 H 5 PhCH 2 CO 2 C 2 H 5
CO 2 CH 3
(d)
+ CH 3 SO 2 O
N CO 2 CH 3 CH 2 CH 2 OSO 2 CH 3
+ O – N O
(e)
Et 3 N
CH 3 SO 2 Cl SO 2
H
CHN N
(f)
Ph N
N N
PhN 3 + NCH CHPh
N
Ph
(g)
O CH 3
(CH 3 ) 2 CHCHOCH 2 CH C(CH 3 ) 2 1) LiNR 2 (CH 3 ) 2 CHC CCH CH 2
2) H 3 O +
N C CH 3
(h) O O OCH 3
110°C
+ CH 2 C(OCH 3 ) 2
CO 2 CH 3 CO 2 CH 3