Page 637 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 637
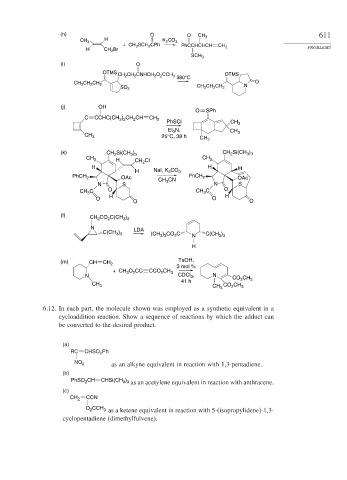
(h) O O CH 3 611
H
CH 3 K 2 CO 3
+ CH 3 SCH 2 CPh PhCCHCHCH
H CH 2 Br CH 2 PROBLEMS
SCH 3
(i) O
OTMS
CH 2 CH 2 CNHCH 2 O 2 CCH 3 OTMS
380°C
O
CH 3 CH 2 CH 2 N
CH 3 CH 2 CH 2
SO 2
(j) OH
O SPh
C CCHC(CH ) CH CH CH 2
3 2
2
PhSCl CH 3
Et N, CH 3
3
CH 3 25°C, 38 h
CH 3
(k) CH Si(CH ) CH Si(CH )
3 3
3 3
2
2
CH 3 H CH 2 Cl CH 3
H H H
H NaI, K 2 CO 3
PhCH 2 OAc CH CN PhCH 2 OAc
N S 3 N S
C O C O
CH 3 CH 3
O H O H
O O
(l)
CH CO C(CH )
2
2
3 3
N LDA
C(CH ) (CH ) CO C N C(CH )
3 3
3 3
3 3
2
H
(m) CH CH TsOH,
2
3 mol %
+ CH O CC CCO CH 3
2
2
3
N CDCl 3 , N CO CH
41 h 2 3
CH 3 CH 3 CO 2 CH 3
6.12. In each part, the molecule shown was employed as a synthetic equivalent in a
cycloaddition reaction. Show a sequence of reactions by which the adduct can
be converted to the desired product.
(a)
RC CHSO Ph
2
NO 2
as an alkyne equivalent in reaction with 1,3-pentadiene.
(b)
PhSO CH CHSi(CH )
2
3 3 as an acetylene equivalent in reaction with anthracene.
(c)
CH 2 CCN
O CCH 3 as a ketene equivalent in reaction with 5-(isopropylidene)-1,3-
2
cyclopentadiene (dimethylfulvene).