Page 638 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 638
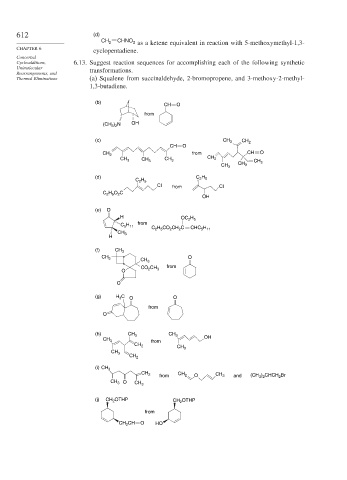
612 (d)
CH 2 CHNO 2 as a ketene equivalent in reaction with 5-methoxymethyl-1,3-
CHAPTER 6
cyclopentadiene.
Concerted
Cycloadditions, 6.13. Suggest reaction sequences for accomplishing each of the following synthetic
Unimolecular
Rearrangements, and transformations.
Thermal Eliminations (a) Squalene from succinaldehyde, 2-bromopropene, and 3-methoxy-2-methyl-
1,3-butadiene.
(b)
CH O
from
(CH 3 ) 2 N OH
(c) CH 3 CH 2
CH O
from CH O
CH 2
CH 2
CH 3 CH 3 CH 3
CH 2
CH 3
CH 3
(d) C 2 H 5
C 2 H 5
Cl from Cl
C 2 H 5 O 2 C
OH
(e) O
H OC 2 H 5
from
C 5 H 11
C 2 H 5 CO 2 CH 2 C CHC 5 H 11
CH 3
H
(f) CH 3
O
CH 3
CH 3
from
O CO 2 CH 3
O
(g) H 3 C O O
from
O
(h) CH 3 CH 3
OH
CH 3
from
CH 2
CH 3
CH 3
CH 2
(i) CH 3
CH 2 CH 2 CH 3 (CH 3 ) 2 CHCH 2 Br
from O and
CH 3 O
CH 3
(j) CH 2 OTHP CH 2 OTHP
from
CH 2 CH O HO