Page 631 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 631
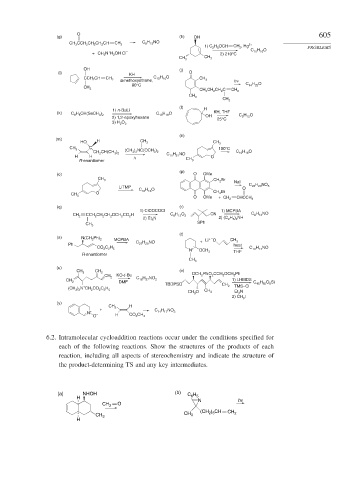
O 605
(g) (h) OH
CH 3 CCH 2 CH 2 CH 2 CH CH 2 C 8 H 15 NO
1) C 2 H 5 OCH CH 2 , Hg 2+ PROBLEMS
C 10 H 16 O
+
+ CH 3 N H 2 OH Cl – 2) 210°C
CH 3
CH 3
OH ( j)
(i) O
KH
CCH 2 CH CH 2 C 10 H 16 O CH 3
dimethoxyethane, h ν
80°C C 14 H 22 O
CH 3
CH 2 CH 2 CH 2 C CH 2
CH 3
CH 3
(l)
1) n -BuLi H
(k) C 13 H 18 O KH, THF
C 6 H 5 CH(SeCH 3 ) 2 OH C 9 H 12 O
2) 1,2- epoxyhexane
25°C
3) H 2 O 2
(n)
(m)
HO H CH 3 CH 3
C
CH 3 100°C
(CH 3 ) 2 NC(OCH 3 ) 2 C 10 H 16 O
CH 2 CH(CH 3 ) 2 C 11 H 21 NO
H H Δ O
R-enantiomer CH 3
(p)
(o) O OMe
CH 3 CH 2 Br
NaI
LiTMP O C 22 H 20 NO 5
C 10 H 16 O
O CH 2 Br
CH 3
O OMe + CH 2 CHCCH 3
(q) (r)
1) ClCOCOCl 1) MCPBA
CCH 2 CH 2 CH 2 OCH 2 CO 2 H C 8 H 12 O 2 CN C 8 H 13 NO
CH 2
2) Et 3 N 2) (C 2 H 5 ) 2 NH
SPh
CH 3
(t)
(s) N(CH 2 Ph) 2 MCPBA Li + – O
Ph C 27 H 29 NO + CH 3
heat
CO 2 C 2 H 5 + C 10 H 17 NO
N OCH 3 THF
R-enantiomer
CH 3
(u)
CH 3 CH 2 (v)
KO-t -Bu OCH 2 PhO 2 CCH 2 OCH 2 Ph
CH 2
C 16 H 27 NO 2 1) LHMDS
DMF TBDPSO C 45 H 55 O 6 Si
CH 2
+ CH 2 TMS–Cl
(CH 3 ) 2 N CH 2 CO 2 C 2 H 5
CH 3 O CH 3 Et 3 N
2) CH 3 I
(y)
CH 3 H
+ C 14 H 17 NO 3
N +
O – H CO 2 CH 3
6.2. Intramolecular cycloaddition reactions occur under the conditions specified for
each of the following reactions. Show the structures of the products of each
reaction, including all aspects of stereochemistry and indicate the structure of
the product-determining TS and any key intermediates.
(a) NHOH (b) C 6 H 5
H
N h ν
O
CH 2
(CH 2 ) 3 CH
CH 3 CH 2
CH 3
H