Page 729 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 729
intermediates. Soluble Cu(I) salts, particularly the triflate, effect coupling of aryl 705
halides at much lower temperatures and under homogeneous conditions. 94
SECTION 8.1
NO 2 Organocopper
NO 2 Intermediates
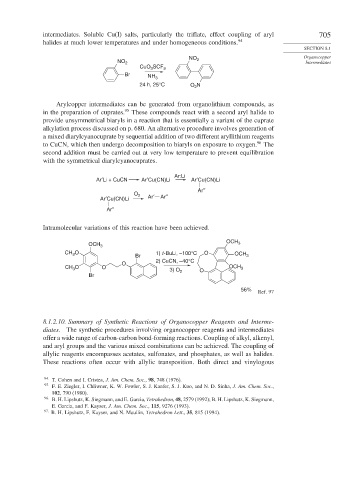
CuO 3 SCF 3
Br NH 3
24 h, 25°C O N
2
Arylcopper intermediates can be generated from organolithium compounds, as
in the preparation of cuprates. 95 These compounds react with a second aryl halide to
provide unsymmetrical biaryls in a reaction that is essentially a variant of the cuprate
alkylation process discussed on p. 680. An alternative procedure involves generation of
a mixed diarylcyanocuprate by sequential addition of two different aryllithium reagents
96
to CuCN, which then undergo decomposition to biaryls on exposure to oxygen. The
second addition must be carried out at very low temperature to prevent equilibration
with the symmetrical diarylcyanocuprates.
Ar:Li
Ar′Li + CuCN Ar′Cu(CN)Li Ar′Cu(CN)Li
Ar″
O 2 Ar′ Ar″
Ar′Cu(CN)Li
Ar″
Intramolecular variations of this reaction have been achieved.
OCH
OCH 3 3
CH O Br 1) t -BuLi, –100°C O OCH 3
3
2) CuCN, –40°C
O
O OCH
CH 3 O 3
3) O 2 O
Br
56%
Ref. 97
8.1.2.10. Summary of Synthetic Reactions of Organocopper Reagents and Interme-
diates. The synthetic procedures involving organocopper reagents and intermediates
offer a wide range of carbon-carbon bond-forming reactions. Coupling of alkyl, alkenyl,
and aryl groups and the various mixed combinations can be achieved. The coupling of
allylic reagents encompasses acetates, sulfonates, and phosphates, as well as halides.
These reactions often occur with allylic transposition. Both direct and vinylogous
94
T. Cohen and I. Cristea, J. Am. Chem. Soc., 98, 748 (1976).
95
F. E. Ziegler, I. Chliwner, K. W. Fowler, S. J. Kanfer, S. J. Kuo, and N. D. Sinha, J. Am. Chem. Soc.,
102, 790 (1980).
96 B. H. Lipshutz, K. Siegmann, and E. Garcia, Tetrahedron, 48, 2579 (1992); B. H. Lipshutz, K. Siegmann,
E. Garcia, and F. Kayser, J. Am. Chem. Soc., 115, 9276 (1993).
97
B. H. Lipshutz, F. Kayser, and N. Maullin, Tetrahedron Lett., 35, 815 (1994).