Page 726 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 726
83
702 The role of BF catalysis in the conjugate addition was also explored. Inclusion
3
of BF results in a considerable stabilization of the reaction complex, but there is also
3
CHAPTER 8
a lowered barrier for the rate-determining reductive elimination. This suggests that
Reactions Involving BF functions primarily at the Cu(III) stage by facilitating the decomposition of the
Transition Metals 3
Cu(III) intermediate.
R RCu I
R[–CuR] – R Cu R BF 3 R Cu III F fast R BF 3
BF 2 O –
:
O O O
A similar sequence of intermediates and TSs was found for the reaction of
cyclohexenone. 82 In this case, both axial and equatorial approaches were examined.
At the crucial rate- and product-determining TS for C−C bond formation, the axial
pathway is favored by 1.7 kcal/mol, in agreement with experimental results from
conformationally biased cyclohexenones. Nearly all of the difference is due to factors
in the cyclohexenone ring and transferring methyl group. This result suggests that
analysis of stereoselectivity of cuprate conjugate additions should focus on the relative
energies of the competing TS for the C−C bond-forming step. These computational
studies comport well with a variety of product, kinetic, and spectroscopic studies
that have been applied to determining the mechanism of organocuprates and related
reagents. 84
Visual models and additional information on Organocuprate Intermediates can
be found in the Digital Resource available at: Springer.com/carey-sundberg.
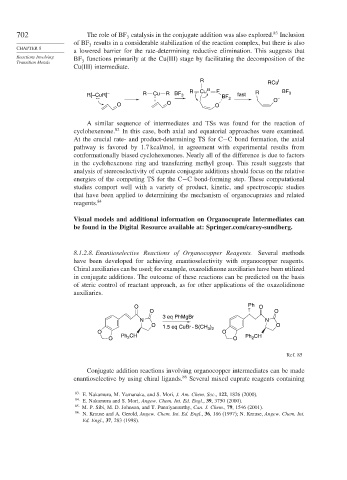
8.1.2.8. Enantioselective Reactions of Organocopper Reagents. Several methods
have been developed for achieving enantioselectivity with organocopper reagents.
Chiral auxiliaries can be used; for example, oxazolidinone auxiliaries have been utilized
in conjugate additions. The outcome of these reactions can be predicted on the basis
of steric control of reactant approach, as for other applications of the oxazolidinone
auxiliaries.
O Ph O
O O
3 eq PhMgBr
N N
O 1.5 eq CuBr - S(CH ) O
O 3 2 O
Ph CH Ph CH
O 2 O 2
Ref. 85
Conjugate addition reactions involving organocopper intermediates can be made
enantioselective by using chiral ligands. 86 Several mixed cuprate reagents containing
83
E. Nakamura, M. Yamanaka, and S. Mori, J. Am. Chem. Soc., 122, 1826 (2000).
84 E. Nakamura and S. Mori, Angew. Chem. Int. Ed. Engl., 39, 3750 (2000).
85 M. P. Sibi, M. D. Johnson, and T. Punniyamurthy, Can. J. Chem., 79, 1546 (2001).
86
N. Krause and A. Gerold, Angew. Chem. Int. Ed. Engl., 36, 186 (1997); N. Krause, Angew. Chem. Int.
Ed. Engl., 37, 283 (1998).