Page 721 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 721
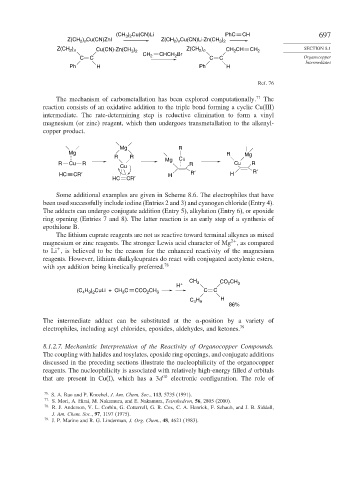
(CH 3 ) 2 Cu(CN)Li PhC CH 697
Z(CH 2 ) n Cu(CN)ZnI Z(CH 2 ) n Cu(CN)Li·Zn(CH 3 ) 2
)
Z(CH 2 ) n Cu(CN)·Zn(CH 3 2 Z(CH 2 ) n CH 2 CH CH 2 SECTION 8.1
CH CHCH Br
C C 2 2 C C Organocopper
Intermediates
Ph H Ph H
Ref. 76
The mechanism of carbometallation has been explored computationally. 77 The
reaction consists of an oxidative addition to the triple bond forming a cyclic Cu(III)
intermediate. The rate-determining step is reductive elimination to form a vinyl
magnesium (or zinc) reagent, which then undergoes transmetallation to the alkenyl-
copper product.
Mg R
Mg R Mg
R R Mg Cu
R Cu R R Cu R
Cu
HC CR′ H R′ H R′
HC CR′
Some additional examples are given in Scheme 8.6. The electrophiles that have
been used successfully include iodine (Entries 2 and 3) and cyanogen chloride (Entry 4).
The adducts can undergo conjugate addition (Entry 5), alkylation (Entry 6), or epoxide
ring opening (Entries 7 and 8). The latter reaction is an early step of a synthesis of
epothilone B.
The lithium cuprate reagents are not as reactive toward terminal alkynes as mixed
2+
magnesium or zinc reagents. The stronger Lewis acid character of Mg , as compared
+
to Li , is believed to be the reason for the enhanced reactivity of the magnesium
reagents. However, lithium dialkylcuprates do react with conjugated acetylenic esters,
with syn addition being kinetically preferred. 78
2
H + CH 3 CO CH 3
(C H ) CuLi + CH C CCO CH 3 C C
4 9 2
2
3
C H H
4 9
86%
The intermediate adduct can be substituted at the -position by a variety of
electrophiles, including acyl chlorides, epoxides, aldehydes, and ketones. 79
8.1.2.7. Mechanistic Interpretation of the Reactivity of Organocopper Compounds.
The coupling with halides and tosylates, epoxide ring openings, and conjugate additions
discussed in the preceding sections illustrate the nucleophilicity of the organocopper
reagents. The nucleophilicity is associated with relatively high-energy filled d orbitals
that are present in Cu(I), which has a 3d 10 electronic configuration. The role of
76 S. A. Rao and P. Knochel, J. Am. Chem. Soc., 113, 5735 (1991).
77
S. Mori, A. Hirai, M. Nakamura, and E. Nakamura, Tetrahedron, 56, 2805 (2000).
78 R. J. Anderson, V. L. Corbin, G. Cotterrell, G. R. Cox, C. A. Henrick, F. Schaub, and J. B. Siddall,
J. Am. Chem. Soc., 97, 1197 (1975).
79
J. P. Marino and R. G. Linderman, J. Org. Chem., 48, 4621 (1983).