Page 723 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 723
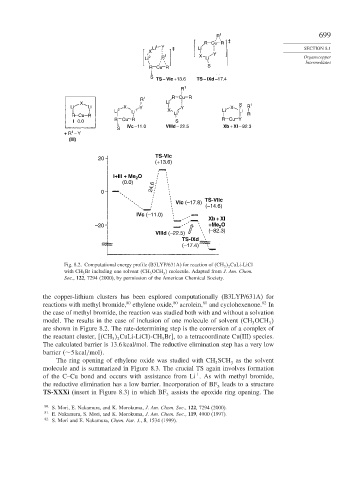
R 1 699
‡
R Cu R
Li 1 Y ‡ Li SECTION 8.1
X Y
Li 2 R 1 X Li Organocopper
Intermediates
R Cu R S
S
TS – VIc +13.6 TS – IXd –17.4
R 1
R 1 R Cu R
X Li S
Li Li X Y Y X R 1
Li 2 Li 1 X 1 Li Li
R Cu R Li R
I 0.0 R Cu R S R Cu Y
IVc –11.0 VIIId – 22.5 Xb + XI – 82.3
S
1
+ R – Y
(III)
20 TS-VIc
(+13.6)
I+III + Me O
2
(0.0)
0 24.6
VIc (–17.8) TS-VIIc
(–14.6)
IVc (–11.0)
Xb + XI
–20 +Me O
2
(–82.3)
VIIId (–22.5)
TS-IXd
(–17.4)
Fig. 8.2. Computational energy profile (B3LYP/631A) for reaction of CH 3 2 CuLi-LiCl
with CH 3 Br including one solvent CH 3 OCH molecule. Adapted from J. Am. Chem.
3
Soc., 122, 7294 (2000), by permission of the American Chemical Society.
the copper-lithium clusters has been explored computationally (B3LYP/631A) for
81
80
80
82
reactions with methyl bromide, ethylene oxide, acrolein, and cyclohexenone. In
the case of methyl bromide, the reaction was studied both with and without a solvation
model. The results in the case of inclusion of one molecule of solvent CH OCH
3
3
are shown in Figure 8.2. The rate-determining step is the conversion of a complex of
the reactant cluster, CH CuLi-LiCl -CH Br
, to a tetracoordinate Cu(III) species.
3 2
3
The calculated barrier is 13.6 kcal/mol. The reductive elimination step has a very low
barrier ∼5kcal/mol .
The ring opening of ethylene oxide was studied with CH SCH as the solvent
3
3
molecule and is summarized in Figure 8.3. The crucial TS again involves formation
+
of the C–Cu bond and occurs with assistance from Li . As with methyl bromide,
the reductive elimination has a low barrier. Incorporation of BF leads to a structure
3
TS-XXXi (insert in Figure 8.3) in which BF assists the epoxide ring opening. The
3
80
S. Mori, E. Nakamura, and K. Morokuma, J. Am. Chem. Soc., 122, 7294 (2000).
81 E. Nakamura, S. Mori, and K. Morokuma, J. Am. Chem. Soc., 119, 4900 (1997).
82
S. Mori and E. Nakamura, Chem. Eur. J., 5, 1534 (1999).