Page 724 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 724
700
CHAPTER 8
Reactions Involving
Transition Metals
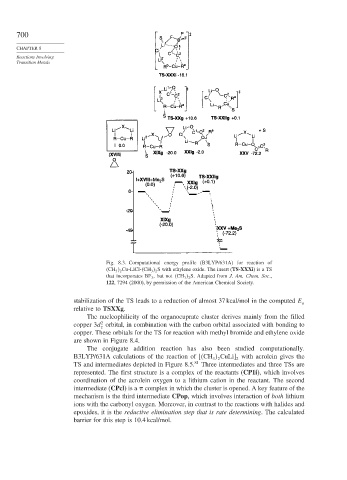
Fig. 8.3. Computational energy profile (B3LYP/631A) for reaction of
CH 3 2 Cu-LiCl- CH 3 2 S with ethylene oxide. The insert (TS-XXXi)isaTS
that incorporates BF 3 , but not CH 3 2 S. Adapted from J. Am. Chem. Soc.,
122, 7294 (2000), by permission of the American Chemical Society.
stabilization of the TS leads to a reduction of almost 37 kcal/mol in the computed E
a
relative to TSXXg.
The nucleophilicity of the organocuprate cluster derives mainly from the filled
2
copper 3d orbital, in combination with the carbon orbital associated with bonding to
z
copper. These orbitals for the TS for reaction with methyl bromide and ethylene oxide
are shown in Figure 8.4.
The conjugate addition reaction has also been studied computationally.
B3LYP/631A calculations of the reaction of CH CuLi
with acrolein gives the
2
3 2
TS and intermediates depicted in Figure 8.5. 81 Three intermediates and three TSs are
represented. The first structure is a complex of the reactants (CP1i), which involves
coordination of the acrolein oxygen to a lithium cation in the reactant. The second
intermediate (CPcl)isa complex in which the cluster is opened. A key feature of the
mechanism is the third intermediate CPop, which involves interaction of both lithium
ions with the carbonyl oxygen. Moreover, in contrast to the reactions with halides and
epoxides, it is the reductive elimination step that is rate determining. The calculated
barrier for this step is 10.4 kcal/mol.