Page 814 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 814
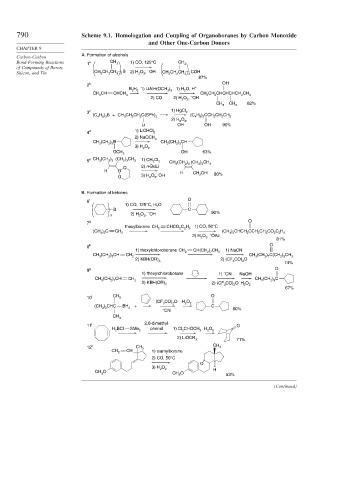
790 Scheme 9.1. Homologation and Coupling of Organoboranes by Carbon Monoxide
and Other One-Carbon Donors
CHAPTER 9
A. Formation of alcohols
Carbon-Carbon
Bond-Forming Reactions 1 a CH 3 1) CO, 125°C CH 3
of Compounds of Boron, –
Silicon, and Tin CH CH CH 3 3 B 2) H O 2 , OH CH CH CH 3 3 COH
2
2
3
2
3
87%
2 b OH
B H 1) LiAlH(OCH ) 1) H O, H +
CH CH CHCH 2 6 3 3 2 CH CH 2 CHCHCHCH 2 CH 3
3 3 – 3
2) CO 2) H O , OH
2
2
CH CH 82%
3 3
3 c 1) HgCl 2
(C H ) B + CH CH CH C(SPh) (C H ) CCH CH CH
4 9 3 3 2 2 2 4 9 2 2 2 3
2) H O , 2
2
–
Li OH OH 90%
4 d 1) LiCHCl 2
2) NaOCH
CH (CH ) B 3 CH (CH ) CH
3 2 5 3 2 5
3) H O 2
2
OH 63%
OCH 3
e CH (CH ) (CH ) CH 1) CH Cl
5 3 2 3 2 3 3 2 2 CH (CH ) 2 3 (CH 2 3 ) CH 3
3
O 2) n-BuLi
H B H CH OH
O , OH 2 80%
O 3) H 2 2
B. Formation of ketones
f O
6
1) CO, 125°C, H O
2
B C
–
O , OH 90%
3 2) H 2 2
7 g O
thexylborane CH CHCO C H 1) CO, 50°C
) CHCH CCH CH CO C H
(CH ) C CH 2 2 2 5 (CH 3 2
3 2 2 – 2 2 2 2 2 5
2
2) H 2 O , OAc
81%
h O
8
1) thexylchloroborane CH CH(CH ) CH 1) NaCN
CH (CH ) CH CH 2 2 7 3 CH 3 (CH ) C(CH ) CH
3 2 5 2 2 7 2 9 3
CO) O
2) KBH(OR) 3 2) (CF 3
2
74%
9 g O
–
1) thexylchloroborane 1) CN NaOH
CH (CH ) CH CH CH (CH ) C
3 2 7 2 3 2 9
2) KBH(OR) 3 2) (CF 3 CO) O H 2 O 2
2
67%
10 i CH 3 O
(CF CO) O H O
(CH ) CHC BH + 3 2 2 2 C
3 2 2 80%
–
CN
CH
3
11 j 2,6-dimethyl- O
H BCl SMe phenol 1) Cl CHOCH H O
2 2 2 3 2 2
2) LiOCR 3 71%
12 k CH CH 3
CH CH 3 1) siamylborane
2
2) CO, 50°C
O
O
3) H 2 2 H
CH O CH 3 O 53%
3
(Continued)