Page 815 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 815
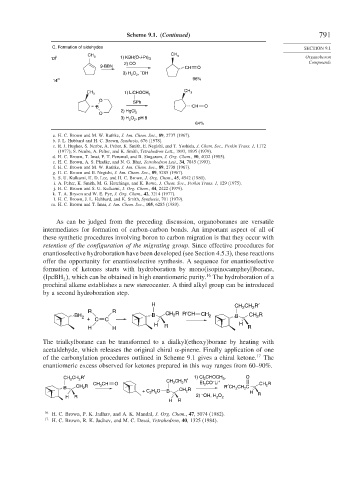
Scheme 9.1. (Continued) 791
C. Formation of aldehydes SECTION 9.1
CH CH 3
13 l 3 1) KBH(O-i-Pr) 3 Organoboron
2) CO Compounds
9-BBN CH O
–
3) H O , OH
2 2
14 m 96%
CH 1) LiCHOCH CH 3
3 3
O SPh
B CH O
2) HgCl
O 2
3) H O , pH 8
2 2
64%
a. H. C. Brown and M. W. Rathke, J. Am. Chem. Soc., 89, 2737 (1967).
b. J. L. Hubbard and H. C. Brown, Synthesis, 676 (1978).
c. R. J. Hughes, S. Ncube, A. Pelter, K. Smith, E. Negishi, and T. Yoshida, J. Chem. Soc., Perkin Trans. 1, 1172
(1977); S. Ncube, A. Pelter, and K. Smith, Tetrahedron Lett., 1893, 1895 (1979).
d. H. C. Brown, T. Imai, P. T. Perumal, and B. Singaram, J. Org. Chem., 50, 4032 (1985).
e. H. C. Brown, A. S. Phadke, and N. G. Bhat, Tetrahedron Lett., 34, 7845 (1993).
f. H. C. Brown and M. W. Rathke, J. Am. Chem. Soc., 89, 2738 (1967).
g. H. C. Brown and E. Negishi, J. Am. Chem. Soc., 89, 5285 (1967).
h. S. U. Kulkarni, H. D. Lee, and H. C. Brown, J. Org. Chem., 45, 4542 (1980).
i. A. Pelter, K. Smith, M. G. Hutchings, and K. Rowe, J. Chem. Soc., Perkin Trans. 1, 129 (1975).
j. H. C. Brown and S. U. Kulkarni, J. Org. Chem., 44, 2422 (1979).
k. T. A. Bryson and W. E. Pye, J. Org. Chem., 42, 3214 (1977).
l. H. C. Brown, J. L. Hubbard, and K. Smith, Synthesis, 701 (1979).
m. H. C. Brown and T. Imai, J. Am. Chem. Soc., 105, 6285 (1983).
As can be judged from the preceding discussion, organoboranes are versatile
intermediates for formation of carbon-carbon bonds. An important aspect of all of
these synthetic procedures involving boron to carbon migration is that they occur with
retention of the configuration of the migrating group. Since effective procedures for
enantioselective hydroboration have been developed (see Section 4.5.3), these reactions
offer the opportunity for enantioselective synthesis. A sequence for enantioselective
formation of ketones starts with hydroboration by mono(isopinocampheyl)borane,
16
IpcBH , which can be obtained in high enantiomeric purity. The hydroboration of a
2
prochiral alkene establishes a new stereocenter. A third alkyl group can be introduced
by a second hydroboration step.
H CH R′
CH 2 2
R R
BH 2 + C C B CH 2 R R′CH CH 2 B CH 2 R
H H
H H R R
The trialkylborane can be transformed to a dialkyl(ethoxy)borane by heating with
acetaldehyde, which releases the original chiral -pinene. Finally application of one
of the carbonylation procedures outlined in Scheme 9.1 gives a chiral ketone. 17 The
enantiomeric excess observed for ketones prepared in this way ranges from 60–90%.
CH R′ 1) Cl CHOCH , O
CH 2 2 CH CH R′ 2 3
–
CH CH O 2 2 Et CO Li + CH R
3
B CH R 3 CH R R′CH 2 CH 2 C 2
2
+ C H O B 2 H R
5
2
–
H R 2) OH, H O 2
2
H R
16 H. C. Brown, P. K. Jadhav, and A. K. Mandal, J. Org. Chem., 47, 5074 (1982).
17
H. C. Brown, R. K. Jadhav, and M. C. Desai, Tetrahedron, 40, 1325 (1984).