Page 812 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 812
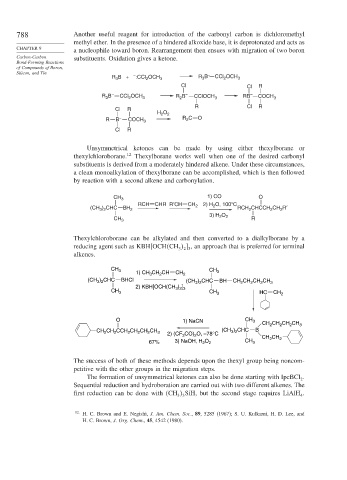
788 Another useful reagent for introduction of the carbonyl carbon is dichloromethyl
methyl ether. In the presence of a hindered alkoxide base, it is deprotonated and acts as
CHAPTER 9
a nucleophile toward boron. Rearrangement then ensues with migration of two boron
Carbon-Carbon substituents. Oxidation gives a ketone.
Bond-Forming Reactions
of Compounds of Boron,
Silicon, and Tin –
–
R B + :CCl OCH 3 R B CCl OCH 3
3
2
3
2
Cl Cl R
– – –
3 2 3 2 3 COCH 3
R B CCl OCH R B CClOCH RB
R Cl R
Cl R
2
H O 2
R B – COCH 3 R 2 C O
Cl R
Unsymmetrical ketones can be made by using either thexylborane or
thexylchloroborane. 12 Thexylborane works well when one of the desired carbonyl
substituents is derived from a moderately hindered alkene. Under these circumstances,
a clean monoalkylation of thexylborane can be accomplished, which is then followed
by reaction with a second alkene and carbonylation.
1) CO O
CH 3
RCH CHR R′CH CH O, 100° C
(CH ) CHC BH 2 2 2) H 2 RCH CHCCH CH R′
2
2
2
3 2
3) H 2 O 2
CH 3 R
Thexylchloroborane can be alkylated and then converted to a dialkylborane by a
reducing agent such as KBH OCH CH , an approach that is preferred for terminal
3 2 3
alkenes.
CH CH
3
1) CH CH CH CH 2 3
3
2
(CH ) CHC BHCl (CH ) CHC BH CH CH CH CH 3
3 2
2
3 2
2
2
) ]
2) KBH[OCH(CH 3 2 3
CH 3 CH 3 HC CH 2
O 1) NaCN CH 3
CH CH CH CH 3
2
2
2
(CH ) CHC B
CH 2 CH 2 CCH 2 CH 2 CH 2 CH 3 CO) O, –78°C 3 2
2) (CF 3 2 CH CH
67% 3) NaOH, H O 2 CH 3 2 2
2
The success of both of these methods depends upon the thexyl group being noncom-
petitive with the other groups in the migration steps.
The formation of unsymmetrical ketones can also be done starting with IpcBCl .
2
Sequential reduction and hydroboration are carried out with two different alkenes. The
first reduction can be done with CH SiH, but the second stage requires LiAlH .
3 3
4
12
H. C. Brown and E. Negishi, J. Am. Chem. Soc., 89, 5285 (1967); S. U. Kulkarni, H. D. Lee, and
H. C. Brown, J. Org. Chem., 45, 4542 (1980).