Page 936 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 936
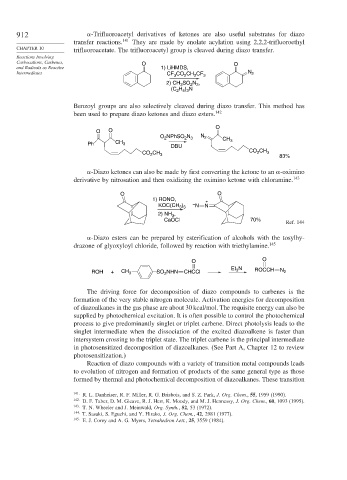
912 -Trifluoroacetyl derivatives of ketones are also useful substrates for diazo
transfer reactions. 141 They are made by enolate acylation using 2,2,2-trifluoroethyl
CHAPTER 10 trifluoroacetate. The trifluoroacetyl group is cleaved during diazo transfer.
Reactions Involving
Carbocations, Carbenes, O O
and Radicals as Reactive 1) LiHMDS,
Intermediates CF CO CH CF 3 N 2
2
2
3
2) CH SO N ,
2 3
3
(C H ) N
2 5 3
Benzoyl groups are also selectively cleaved during diazo transfer. This method has
been used to prepare diazo ketones and diazo esters. 142
O
O O
O NPhSO N N 2 CH
2
2 3
Ph CH 3 3
DBU
2
CO 2 CH 3 CO CH 3 83%
-Diazo ketones can also be made by first converting the ketone to an -oximino
derivative by nitrosation and then oxidizing the oximino ketone with chloramine. 143
O O
1) RONO, +
KOC(CH ) – N N
3 3
2) NH ,
3
CaOCl 70%
Ref. 144
-Diazo esters can be prepared by esterification of alcohols with the tosylhy-
drazone of glyoxyloyl chloride, followed by reaction with triethylamine. 145
O O
Et N ROCCH
ROH + CH 3 SO NHN CHCCl 3 N 2
2
The driving force for decomposition of diazo compounds to carbenes is the
formation of the very stable nitrogen molecule. Activation energies for decomposition
of diazoalkanes in the gas phase are about 30 kcal/mol. The requisite energy can also be
supplied by photochemical excitation. It is often possible to control the photochemical
process to give predominantly singlet or triplet carbene. Direct photolysis leads to the
singlet intermediate when the dissociation of the excited diazoalkene is faster than
intersystem crossing to the triplet state. The triplet carbene is the principal intermediate
in photosensitized decomposition of diazoalkanes. (See Part A, Chapter 12 to review
photosensitization.)
Reaction of diazo compounds with a variety of transition metal compounds leads
to evolution of nitrogen and formation of products of the same general type as those
formed by thermal and photochemical decomposition of diazoalkanes. These transition
141 R. L. Danheiser, R. F. Miller, R. G. Brisbois, and S. Z. Park, J. Org. Chem., 55, 1959 (1990).
142
D. F. Taber, D. M. Gleave, R. J. Herr, K. Moody, and M. J. Hennessy, J. Org. Chem., 60, 1093 (1995).
143 T. N. Wheeler and J. Meinwald, Org. Synth., 52, 53 (1972).
144 T. Sasaki, S. Eguchi, and Y. Hirako, J. Org. Chem., 42, 2981 (1977).
145
E. J. Corey and A. G. Myers, Tetrahedron Lett., 25, 3559 (1984).