Page 941 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 941
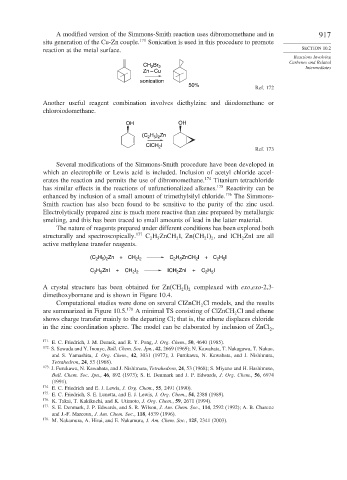
A modified version of the Simmons-Smith reaction uses dibromomethane and in 917
situ generation of the Cu-Zn couple. 171 Sonication is used in this procedure to promote
reaction at the metal surface. SECTION 10.2
Reactions Involving
Carbenes and Related
CH Br 2 Intermediates
2
Zn – Cu
sonication
50%
Ref. 172
Another useful reagent combination involves diethylzinc and diiodomethane or
chloroiodomethane.
OH OH
(C H ) Zn
2 5 2
ClCH 2 I
Ref. 173
Several modifications of the Simmons-Smith procedure have been developed in
which an electrophile or Lewis acid is included. Inclusion of acetyl chloride accel-
erates the reaction and permits the use of dibromomethane. 174 Titanium tetrachloride
has similar effects in the reactions of unfunctionalized alkenes. 175 Reactivity can be
enhanced by inclusion of a small amount of trimethylsilyl chloride. 176 The Simmons-
Smith reaction has also been found to be sensitive to the purity of the zinc used.
Electrolytically prepared zinc is much more reactive than zinc prepared by metallurgic
smelting, and this has been traced to small amounts of lead in the latter material.
The nature of reagents prepared under different conditions has been explored both
structurally and spectroscopically. 177 C H ZnCH I, Zn(CH I) , and ICH ZnI are all
2
2
2
2
5
2
active methylene transfer reagents.
(C H ) Zn + CH 2 2 C 2 5 2 C H I
I
H ZnCH I +
2 5
2 5 2
H ZnI + CH I ICH ZnI + C H I
C 2 5 2 2 2 2 5
A crystal structure has been obtained for Zn(CH I) complexed with exo,exo-2,3-
2
2
dimethoxybornane and is shown in Figure 10.4.
Computational studies were done on several ClZnCH Cl models, and the results
2
are summarized in Figure 10.5. 178 A minimal TS consisting of ClZnCH Cl and ethene
2
shows charge transfer mainly to the departing Cl; that is, the ethene displaces chloride
in the zinc coordination sphere. The model can be elaborated by inclusion of ZnCl ,
2
171
E. C. Friedrich, J. M. Demek, and R. Y. Pong, J. Org. Chem., 50, 4640 (1985).
172 S. Sawada and Y. Inouye, Bull. Chem. Soc. Jpn., 42, 2669 (1969); N. Kawabata, T. Nakagawa, T. Nakao,
and S. Yamashita, J. Org. Chem., 42, 3031 (1977); J. Furukawa, N. Kawabata, and J. Nishimura,
Tetrahedron, 24, 53 (1968).
173
J. Furukawa, N. Kawabata, and J. Nishimura, Tetrahedron, 24, 53 (1968); S. Miyano and H. Hashimoto,
Bull. Chem. Soc. Jpn., 46, 892 (1973); S. E. Denmark and J. P. Edwards, J. Org. Chem., 56, 6974
(1991).
174 E. C. Friedrich and E. J. Lewis, J. Org. Chem., 55, 2491 (1990).
175
E. C. Friedrich, S. E. Lunetta, and E. J. Lewis, J. Org. Chem., 54, 2388 (1989).
176
K. Takai, T. Kakikuchi, and K. Utimoto, J. Org. Chem., 59, 2671 (1994).
177 S. E. Denmark, J. P. Edwards, and S. R. Wilson, J. Am. Chem. Soc., 114, 2592 (1992); A. B. Charette
and J.-F. Marcoux, J. Am. Chem. Soc., 118, 4539 (1996).
178
M. Nakamura, A. Hirai, and E. Nakamura, J. Am. Chem. Soc., 125, 2341 (2003).