Page 946 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 946
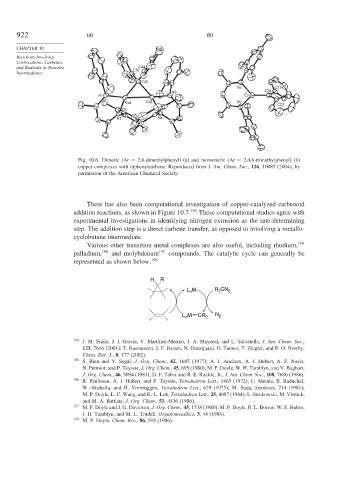
922 (a) (b)
CHAPTER 10
Reactions Involving
Carbocations, Carbenes,
and Radicals as Reactive
Intermediates
Fig. 10.6. Dimeric (Ar = 2,6-dimethylphenyl) (a) and monomeric (Ar = 2,4,6-trimethylphenyl) (b)
copper complexes with diphenylcarbene. Reproduced from J. Am. Chem. Soc., 126, 10085 (2004), by
permission of the American Chemical Society.
There has also been computational investigation of copper-catalyzed carbenoid
addition reactions, as shown in Figure 10.7. 194 These computational studies agree with
experimental investigations in identifying nitrogen extrusion as the rate-determining
step. The addition step is a direct carbene transfer, as opposed to involving a metallo-
cyclobutane intermediate.
Various other transition metal complexes are also useful, including rhodium, 195
palladium, 196 and molybdenum 197 compounds. The catalytic cycle can generally be
represented as shown below. 198
R R
L M R CN 2
2
n
L M CR 2 N 2
n
194 J. M. Fraile, J. I. Garcia, V. Martinez-Merino, J. A. Mayoral, and L. Salvatella, J. Am. Chem. Soc.,
123, 7616 (2001); T. Rasmussen, J. F. Jensen, N. Ostergaard, D. Tanner, T. Ziegler, and P.-O. Norrby,
Chem. Eur. J., 8, 177 (2002).
195 S. Bien and Y. Segal, J. Org. Chem., 42, 1685 (1977); A. J. Anciaux, A. J. Hubert, A. F. Noels,
N. Petiniot, and P. Teyssie, J. Org. Chem., 45, 695 (1980); M. P. Doyle, W. H. Tamblyn, and V. Baghari,
J. Org. Chem., 46, 5094 (1981); D. F. Taber and R. E. Ruckle, Jr., J. Am. Chem. Soc., 108, 7686 (1986).
196
R. Paulissen, A. J. Hubert, and P. Teyssie, Tetrahedron Lett., 1465 (1972); U. Mende, B. Raduchel,
W. Skuballa, and H. Vorbruggen, Tetrahedron Lett., 629 (1975); M. Suda, Synthesis, 714 (1981);
M. P. Doyle, L. C. Wang, and K.-L. Loh, Tetrahedron Lett., 25, 4087 (1984); L. Strekowski, M. Visnick,
and M. A. Battiste, J. Org. Chem., 51, 4836 (1986).
197 M. P. Doyle and J. G. Davidson, J. Org. Chem., 45, 1538 (1980); M. P. Doyle, R. L. Dorow, W. E. Buhro,
J. H. Tamblyn, and M. L. Trudell, Organometallics, 3, 44 (1984).
198
M. P. Doyle, Chem. Rev., 86, 919 (1986).