Page 951 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 951
(a) 927
H H SECTION 10.2
2.13Å H 3.14Å H Reactions Involving
O O O O Carbenes and Related
H O H O Intermediates
O O O O
O O
Rh Rh Rh Rh
H H H
2.94Å O 2.89Å 2.94Å O
O O O O
O O
H H
H H
E = –12.1 E rel = –10.4
rel
(b)
2.98Å
2.90Å
H
H 2.32Å H H
C
H 3 O O O
O H O
H H O
O O O O
O O
Rh Rh H 3 C Rh Rh
H C H H 3 C
3
2.61Å O O O 2.61Å O O O
2.30Å O O
H 3.21Å H
H H
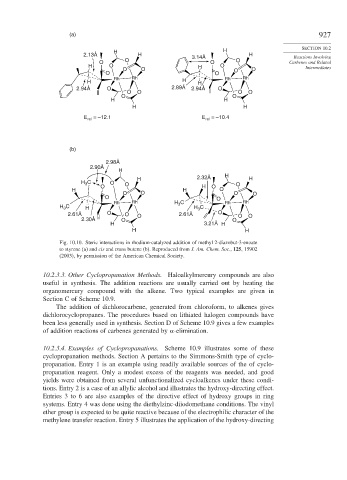
Fig. 10.10. Steric interactions in rhodium-catalyzed addition of methyl 2-diazobut-3-enoate
to styrene (a) and cis and trans butene (b). Reproduced from J. Am. Chem. Soc., 125, 15902
(2003), by permission of the American Chemical Society.
10.2.3.3. Other Cyclopropanation Methods. Haloalkylmercury compounds are also
useful in synthesis. The addition reactions are usually carried out by heating the
organomercury compound with the alkene. Two typical examples are given in
Section C of Scheme 10.9.
The addition of dichlorocarbene, generated from chloroform, to alkenes gives
dichlorocyclopropanes. The procedures based on lithiated halogen compounds have
been less generally used in synthesis. Section D of Scheme 10.9 gives a few examples
of addition reactions of carbenes generated by -elimination.
10.2.3.4. Examples of Cyclopropanations. Scheme 10.9 illustrates some of these
cyclopropanation methods. Section A pertains to the Simmons-Smith type of cyclo-
propanation. Entry 1 is an example using readily available sources of the of cyclo-
propanation reagent. Only a modest excess of the reagents was needed, and good
yields were obtained from several unfunctionalized cycloalkenes under these condi-
tions. Entry 2 is a case of an allylic alcohol and illustrates the hydroxy-directing effect.
Entries 3 to 6 are also examples of the directive effect of hydroxy groups in ring
systems. Entry 4 was done using the diethylzinc-diiodomethane conditions. The vinyl
ether group is expected to be quite reactive because of the electrophilic character of the
methylene transfer reaction. Entry 5 illustrates the application of the hydroxy-directing