Page 953 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 953
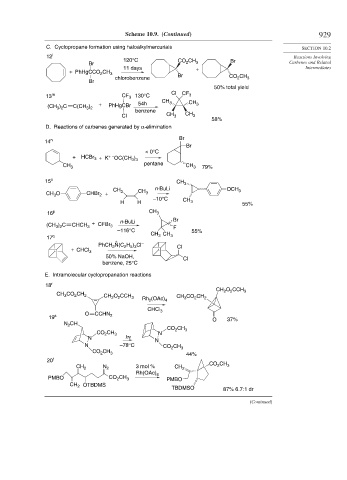
Scheme 10.9. (Continued) 929
C. Cyclopropane formation using haloalkylmercurials SECTION 10.2
12 l Reactions Involving
120°C CO CH Br
Br 2 3 Carbenes and Related
11 days + Intermediates
+ PhHgCCO CH 3
2
chlorobenzene Br CO CH 3
2
Br
50% total yield
13 m CF 3 130°C Cl CF 3
CH 3 CH
(CH ) C C(CH ) + PhHgCBr 54h 3
3 2
3 2
benzene
Cl CH 3 CH 3
58%
D. Reactions of carbenes generated by α-elimination
14 n Br
Br
< 0°C
+ HCBr + K + – OC(CH )
3
3 3
CH 3 pentane CH 3 79%
15 o CH 3
n-BuLi
CH 3 OCH 3
CH O CHBr + CH 3
2
3
–10°C CH
H H 3 55%
16 p CH 3
(CH ) C CHCH 3 + CFBr 3 n-BuLi Br
3 2
F
–116°C 55%
CH CH
17 q 3 3
+
PhCH N(C H ) Cl – Cl
2
2 5 3
+ CHCl 3
50% NaOH, Cl
benzene, 25°C
E. Intramolecular cyclopropanation reactions
18 r
CH O CCH
CH CO CH 2 CH O CCH 3 Rh (OAc) 4 CH CO CH 2 2 2 3
3
2
2
2
3
2
2
CHCl 3
O CCHN
19 s 2 O 37%
N CH
2
CO CH
CO CH 3 N 2 3
2
N hν
N
N –78°C CO CH 3
2
CO CH 3 44%
2
20 t
CH 2 N 2 3 mol % CH 2 CO CH 3
2
Rh(OAc) 4
PMBO CO CH 3 PMBO
2
CH 2 OTBDMS
TBDMSO 87% 6.7:1 dr
(Continued)