Page 950 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 950
926
CHAPTER 10
2.34Å
Reactions Involving
Carbocations, Carbenes, O O O
and Radicals as Reactive
Intermediates
O O
O
Rh
Rh
2.89Å O O
O
O
2.13Å
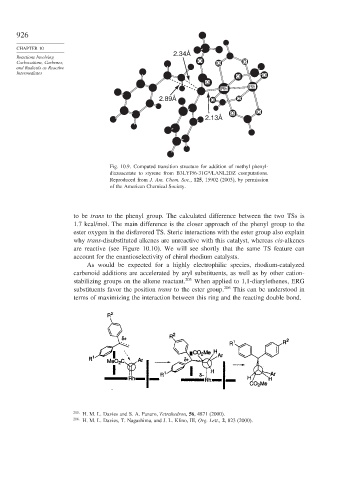
Fig. 10.9. Computed transition structure for addition of methyl phenyl-
diazoacetate to styrene from B3LYP/6-31G*/LANL2DZ computations.
Reproduced from J. Am. Chem. Soc., 125, 15902 (2003), by permission
of the American Chemical Society.
to be trans to the phenyl group. The calculated difference between the two TSs is
1.7 kcal/mol. The main difference is the closer approach of the phenyl group to the
ester oxygen in the disfavored TS. Steric interactions with the ester group also explain
why trans-disubstituted alkenes are unreactive with this catalyst, whereas cis-alkenes
are reactive (see Figure 10.10). We will see shortly that the same TS feature can
account for the enantioselectivity of chiral rhodium catalysts.
As would be expected for a highly electrophilic species, rhodium-catalyzed
carbenoid additions are accelerated by aryl substituents, as well as by other cation-
stabilizing groups on the alkene reactant. 205 When applied to 1,1-diarylethenes, ERG
substituents favor the position trans to the ester group. 206 This can be understood in
terms of maximizing the interaction between this ring and the reacting double bond.
205 H. M. L. Davies and S. A. Panaro, Tetrahedron, 56, 4871 (2000).
206
H. M. L. Davies, T. Nagashima, and J. L. Klino, III, Org. Lett., 2, 823 (2000).