Page 948 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 948
924 Since the additions are normally stereospecific with respect to the alkene, if an open-
chain intermediate is involved it must collapse to product more rapidly than single-bond
CHAPTER 10 rotations that would destroy the stereoselectivity.
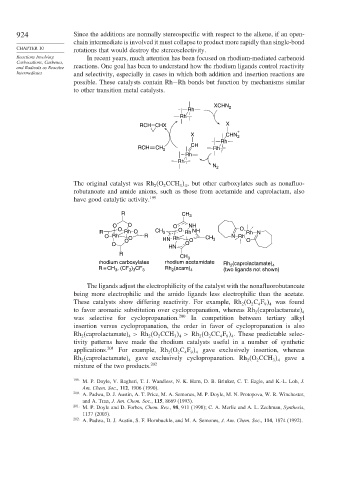
Reactions Involving In recent years, much attention has been focused on rhodium-mediated carbenoid
Carbocations, Carbenes,
and Radicals as Reactive reactions. One goal has been to understand how the rhodium ligands control reactivity
Intermediates and selectivity, especially in cases in which both addition and insertion reactions are
possible. These catalysts contain Rh−Rh bonds but function by mechanisms similar
to other transition metal catalysts.
XCHN
Rh 2
Rh
RCH CHX X
+
X CHN 2
Rh
CH
RCH CH 2 Rh
Rh
Rh
N 2
The original catalyst was Rh (O CCH , but other carboxylates such as nonafluo-
2
2
3 4
robutanoate and amide anions, such as those from acetamide and caprolactam, also
have good catalytic activity. 199
R CH 3
O O O NH
R O Rh O CH 3 O Rh NH O Rh N
O Rh O R N Rh
O HN Rh O CH 3 O
O O
HN
R
CH 3
rhodium carboxylates rhodium acetamidate Rh (caprolactamate) 4
2
R = CH , (CF ) CF 3 Rh (acam) 4 (two ligands not shown)
2
2 3
3
The ligands adjust the electrophilicity of the catalyst with the nonafluorobutanoate
being more electrophilic and the amido ligands less electrophilic than the acetate.
These catalysts show differing reactivity. For example, Rh (O C F was found
2
4 9 4
2
to favor aromatic substitution over cyclopropanation, whereas Rh (caprolactamate) 4
2
was selective for cyclopropanation. 200 In competition between tertiary alkyl
insertion versus cyclopropanation, the order in favor of cyclopropanation is also
Rh (caprolactamate) > Rh (O CCH > Rh (O CC F . These predictable selec-
2
2
2
4
4 9 4
2
2
3 4
tivity patterns have made the rhodium catalysts useful in a number of synthetic
applications. 201 For example, Rh (O C F gave exclusively insertion, whereas
2
4 9 4
2
Rh (caprolactamate) 4 gave exclusively cyclopropanation. Rh (O CCH gave a
3 4
2
2
2
mixture of the two products. 202
199 M. P. Doyle, V. Bagheri, T. J. Wandless, N. K. Harn, D. B. Brinker, C. T. Eagle, and K.-L. Loh, J.
Am. Chem. Soc., 112, 1906 (1990).
200
A. Padwa, D. J. Austin, A. T. Price, M. A. Semones, M. P. Doyle, M. N. Protopova, W. R. Winchester,
and A. Tran, J. Am. Chem. Soc., 115, 8669 (1993).
201 M. P. Doyle and D. Forbes, Chem. Rev., 98, 911 (1998); C. A. Merlic and A. L. Zechman, Synthesis,
1137 (2003).
202
A. Padwa, D. J. Austin, S. F. Hornbuckle, and M. A. Semones, J. Am. Chem. Soc., 114, 1874 (1992).