Page 944 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 944
920
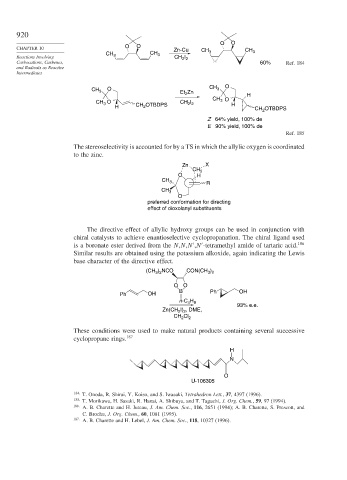
O O
O O
CHAPTER 10 Zn-Cu CH CH
CH CH 3 3 3
I
Reactions Involving 3 CH 2 2
Carbocations, Carbenes, 60% Ref. 184
and Radicals as Reactive
Intermediates
CH 3 O Et Zn CH 3 O H
2
CH 3 O
CH 3 O CH I H
2 2
H CH 2 OTBDPS CH OTBDPS
2
Z 64% yield, 100% de
E 90% yield, 100% de
Ref. 185
The stereoselectivity is accounted for by a TS in which the allylic oxygen is coordinated
to the zinc.
Zn X
CH 2
O H
CH 3 R
CH 3
O
preferred conformation for directing
effect of dioxolanyl substituents
The directive effect of allylic hydroxy groups can be used in conjunction with
chiral catalysts to achieve enantioselective cyclopropanation. The chiral ligand used
is a boronate ester derived from the N,N,N ,N -tetramethyl amide of tartaric acid. 186
Similar results are obtained using the potassium alkoxide, again indicating the Lewis
base character of the directive effect.
) NCO
)
(CH 3 2 CON(CH 3 2
O O
B Ph OH
Ph OH
n-C H
4 9
93% e.e.
Zn(CH I) , DME,
2 2
CH 2 Cl 2
These conditions were used to make natural products containing several successive
cyclopropane rings. 187
H
N
O
U-106305
184
T. Onoda, R. Shirai, Y. Koiso, and S. Iwasaki, Tetrahedron Lett., 37, 4397 (1996).
185 T. Morikawa, H. Sasaki, R. Hanai, A. Shibuya, and T. Taguchi, J. Org. Chem., 59, 97 (1994).
186 A. B. Charette and H. Juteau, J. Am. Chem. Soc., 116, 2651 (1994); A. B. Charette, S. Prescott, and
C. Brochu, J. Org. Chem., 60, 1081 (1995).
187
A. B. Charette and H. Lebel, J. Am. Chem. Soc., 118, 10327 (1996).