Page 949 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 949
N 2 CH O O O CH 3 925
CH 3
CH 3
or CH 3 SECTION 10.2
CH 2 CH 3
Reactions Involving
CH 2 CH 3
Carbenes and Related
Intermediates
100% 0%
Rh 2 (O 2 CC 4 F 9 ) 4
56% 44%
Rh 2 (O 2 CCH 3 ) 4
Rh 2 (caprolactamate) 4 0% 100%
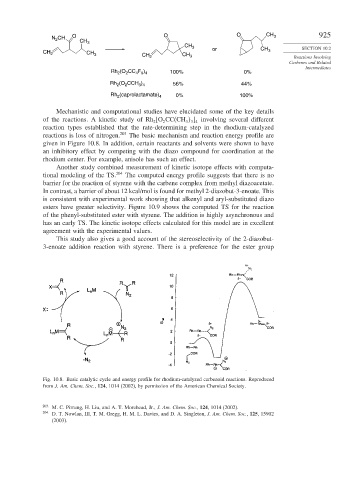
Mechanistic and computational studies have elucidated some of the key details
of the reactions. A kinetic study of Rh [O CC(CH ] involving several different
2 2 3 3 4
reaction types established that the rate-determining step in the rhodium-catalyzed
reactions is loss of nitrogen. 203 The basic mechanism and reaction energy profile are
given in Figure 10.8. In addition, certain reactants and solvents were shown to have
an inhibitory effect by competing with the diazo compound for coordination at the
rhodium center. For example, anisole has such an effect.
Another study combined measurement of kinetic isotope effects with computa-
tional modeling of the TS. 204 The computed energy profile suggests that there is no
barrier for the reaction of styrene with the carbene complex from methyl diazoacetate.
In contrast, a barrier of about 12 kcal/mol is found for methyl 2-diazobut-3-enoate. This
is consistent with experimental work showing that alkenyl and aryl-substituted diazo
esters have greater selectivity. Figure 10.9 shows the computed TS for the reaction
of the phenyl-substituted ester with styrene. The addition is highly asynchronous and
has an early TS. The kinetic isotope effects calculated for this model are in excellent
agreement with the experimental values.
This study also gives a good account of the stereoselectivity of the 2-diazobut-
3-enoate addition reaction with styrene. There is a preference for the ester group
Fig. 10.8. Basic catalytic cycle and energy profile for rhodium-catalyzed carbenoid reactions. Reproduced
from J. Am. Chem. Soc., 124, 1014 (2002), by permission of the American Chemical Society.
203 M. C. Pirrung, H. Liu, and A. T. Morehead, Jr., J. Am. Chem. Soc., 124, 1014 (2002).
204
D. T. Nowlan, III, T. M. Gregg, H. M. L. Davies, and D. A. Singleton, J. Am. Chem. Soc., 125, 15902
(2003).