Page 956 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 956
932 O
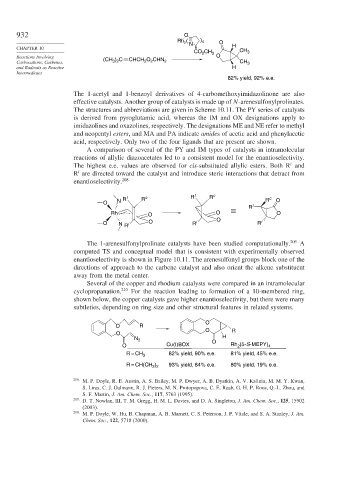
Rh ( N ) 4 O
2
CHAPTER 10 H
CH 3
CO 2 CH 3
Reactions Involving (CH ) C CHCH O CHN O
Carbocations, Carbenes, 3 2 2 2 2 CH 3
and Radicals as Reactive H
Intermediates
82% yield, 92% e.e.
The 1-acetyl and 1-benzoyl derivatives of 4-carbomethoxyimidazolinone are also
effective catalysts. Another group of catalysts is made up of N-arenesulfonylprolinates.
The structures and abbreviations are given in Scheme 10.11. The PY series of catalysts
is derived from pyroglutamic acid, whereas the IM and OX designations apply to
imidazolines and oxazolines, respectively. The designations ME and NE refer to methyl
and neopentyl esters, and MA and PA indicate amides of acetic acid and phenylacetic
acid, respectively. Only two of the four ligands that are present are shown.
A comparison of several of the PY and IM types of catalysts in intramolecular
reactions of allylic diazoacetates led to a consistent model for the enantioselectivity.
t
The highest e.e. values are observed for cis-substituted allylic esters. Both R and
i
R are directed toward the catalyst and introduce steric interactions that detract from
enantioselectivity. 208
O N R t R c R t R c R c O
R t
Rh O O O
O
O N R i O R i R i
The 1-arenesulfonylprolinate catalysts have been studied computationally. 209 A
computed TS and conceptual model that is consistent with experimentally observed
enantioselectivity is shown in Figure 10.11. The arenesulfonyl groups block one of the
directions of approach to the carbene catalyst and also orient the alkene substituent
away from the metal center.
Several of the copper and rhodium catalysts were compared in an intramolecular
cyclopropanation. 210 For the reaction leading to formation of a 10-membered ring,
shown below, the copper catalysts gave higher enantioselectivity, but there were many
subtleties, depending on ring size and other structural features in related systems.
O
O R
O R
O
H
N 2 O
O Cu(I)BOX Rh (5-S-MEPY) 4
2
R = CH 3 82% yield, 90% e.e. 81% yield, 45% e.e.
R = CH(CH ) 93% yield, 84% e.e. 80% yield, 19% e.e.
3 2
208
M. P. Doyle, R. E. Austin, A. S. Bailey, M. P. Dwyer, A. B. Dyatkin, A. V. Kalinin, M. M. Y. Kwan,
S. Liras, C. J. Oalmann, R. J. Pieters, M. N. Protopopova, C. E. Raab, G. H. P. Roos, Q.-L. Zhou, and
S. F. Martin, J. Am. Chem. Soc., 117, 5763 (1995).
209 D. T. Nowlan, III, T. M. Gregg, H. M. L. Davies, and D. A. Singleton, J. Am. Chem. Soc., 125, 15902
(2003).
210
M. P. Doyle, W. Hu, B. Chapman, A. B. Marnett, C. S. Peterson, J. P. Vitale, and S. A. Stanley, J. Am.
Chem. Soc., 122, 5718 (2000).